Cresencio C. Cabuga Jr.,
Jojean Marie D. Pondang, Roy B. Piloton,
Aibie Jel R. Cornites, and Penelope S. Ejada, from the different
institute of the Philippines. wrote a research article about, Endoparasites
in Sardinella lemuru and Glossogobius guiris from Caraga, Philippines.
entitled, Prevalence of Endoparasites in Two Commercially Important Fish
Sardinella lemuru and Glossogobius guiris from Coast of Cabadbaran and Lake
Mainit, Caraga, Philippines. This research paper published by the International Journal of Biosciences (IJB). an open access scholarly research journal on
Biology, under the affiliation of the International Network
For Natural Sciences | INNSpub. an open access
multidisciplinary research journal publisher.
Abstract
Fish often serves as a
staple food and protein source. Most Filipinos depend on fishing activities as
a means of living. Fish parasites are diverse and pervasive organisms affecting
a variety of hosts, including commercially important fish. Despite being
utilized for commercial purposes, limited studies on fish parasitism are done
in the Caraga region and are published in the Philippines. This study is one of
the few conducted on the prevalence of parasites from the coast of Cabadbaran
City and Lake Mainit in Jabonga, Agusan Del Norte, Phils. The two areas were
the major fishing ground for different fish varieties sold in the local market.
Two fish samples were collected: Glossogobius guiris (n=100)
and Sardinella lemuru (n=100). Standardized protocols for fish
parasite investigation were adopted. From the collected fish samples, the
gastrointestinal tract was subjected, then examined and isolated. Of the 200
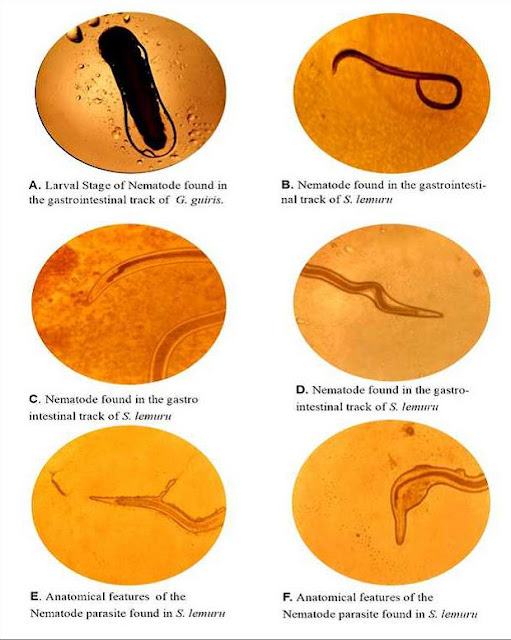
individuals, there were 2 found infected withG. guiris and 8 infected withS.
lemuru. The recorded parasite was identified as Nematodes and was mostly
observed in marine fish species (S. lemuru) when compared to freshwater fish (G.
guiris). This implies that the occurrence of these parasites may vary depending
on the fish type, maturity, diet composition, and location. A significant
difference p=0.0003 was observed in the length-weight and prevalence of
fish parasites and infers that size structure is associated with parasitic
rate. Thus, the importance of conducting fish parasites could give an
understanding of how this ubiquitous organism affects commercially important
fish and awareness to the fish-eating community.
Read more : Medicinal Plant Tradingin Kovilpatti Taluk, South East Tamil Nadu | InformativeBD
Introduction
In the Philippines, fish livelihood activities greatly contribute to the local and national economies. Fishing has produced a large contribution to the country’s development, with the recorded input of an estimated 4.33 billion dollars to the country’s financial resources (BFAR, 2016). Due to this record, there is a need for intensive management of fishery activities to maintain the economic stability of the country. Along with this is the careful assessment of the quality of harvest as its Philippine catch value increases over time (Anticamara and Go, 2016). Fish is successfully marketed according to a range of customer key standards (Saeed et al., 2022), and such requirements are qualities that are largely influenced by environmental factors (Fritrianiet al., 2019).
A prevalent problem in aquatic animals is parasitism which contributes to high mortalities among fish stocks (Edeh & Solomon, 2017). This special interaction between organisms involves a parasite harming a host or limiting its abundance. The impact of these organisms stretches out to an ability to wipe out other species due to their ability to cause wide epidemics, especially for disease-causing parasites (Frainer et al., 2018). Parasites are classified into ectoparasites, parasites on the external part of the host and endoparasites, parasites within the host’s body. Such endoparasites may be located within the flesh and organs of the host organism (Edeh & Solomon, 2017). Flagellates, amoebae, and Haplosporidia are examples of these parasites, to name a few (Lucas etal., 2019). The existence of these harmful organisms affects fish quality such that a study made byRamos, 2020 proved that some of these parasite-infested fish products are commercialized.
Despite their ability to cause diseases, fish are also known to be effective bioindicators. In an evaluation performed by Fierro etal., 2019, fishes were proven to present information on human agricultural activities to which their setting is subjected, as well as the water pollution in the same setting. In addition, fish examination gives information on the health and environmental disturbances by assessment of the inner organs and tolerance. Fish had also been used in a different study by (Gutiérrez and Agudelo, 2020) in assessing the accumulation of coal and mercury in Colombian waters, which gave significant results. Many factors contribute to the results of these bioassessments, namely, the increase in population (Rabadon & Corpuz, 2021), as well as climate change and the pollution that is brought about by the same factor (Wu etal., 2019).
Moreover, the fish-size relationship and the occurrence of parasites in fish products reduce the value and quality to drop, thus changing the marketability of the fish (Abollo etal., 2001; Levsen et al., 2005; Karl, 2008; Llarena-Reino etal., 2013; D’Amico et al. 2014; Llarena-Reino et al., 2015).
Fish parasite infection and pollution develop in ineffective energy and nutrient consumption and an escalation in energy expenditure of fish through respiration, impacting reproduction and growth (Marcogliese and Pietrock, 2011; Khan, 2012; Shea- Donohue et al., 2017; FAO 2020). More importantly, the risk posed by fish-borne parasitic zoonoses to human health (Quiazon, 2015) is estimated to be high, especially since fish is the staple food of fishing communities and eating raw fish is common practice in the region (Soares Magalhães etal., 2014; Tenorio and Molina, 2021).
This study utilized Sardinella lemuru (Tamban) and Glossogobiusguiris (Pidjanga), which is a commercially important fish in the Caraga region and the Philippines as well. Being ranked second in terms of volume in fish production, it is considered to be one of the most abundant fish and an affordable source of protein in the country (Labrador et al., 2021). These fish have a good hold on the economy of the Philippines and can affect the state of that part of the country. However, according to a study (Pohle, 2013), consumers have the trend in food without assessing the quality and information of the said product. In this case, consumers are at risk of diseasecausing factors brought upon by the products they buy due to their lack of awareness and lack of assessment from the food industry (Ziarati etal., 2022). In support of this information, the American Society has found that about 260,000 people have reported being sick from consuming fish products. Likewise, Barrett etal., 2017 also recorded 857 outbreaks causing about 4800 illnesses with hospitalizations and deaths in the same country due to polluted fish.
Further, due to their availability and precise identification of species,
fish assessments prove to be convenient and useful in scientific
investigations. It is also imperative to perform with scientifically proven and
applicable methods along with the locallyobtained samples. More importantly,
there is a need to use a preventive approach in the maintenance of fish health
in our aquatic resources (Assefa & Abunna, 2018). Moreover, Cook, 2017,
infers that the foundation of sustainability rests upon three factors which are
social, economic, and environmental. There were no studies conducted in the
region utilizing the fish samples. Thus, the study aims to provide baseline
information to address the gap. Lastly, the focus of the study is to determine
the prevalence of endoparasites in Sardinella lemuru and Glossogobius guiris.
While this also touches on the protection and conservation of our water
resources as well as the health of the fish-consuming community.
Reference
Aas E, Beyer J, Goksoyr A. 2000. Fixed Wavelength Fluorescence (FF) of Bile as a Monitoring Tool for Polyaromatic Hydrocarbon Exposure in Fish: An Evaluation of Compound Specificity, Inner Filter Effect and Signal Interpretation. Biomarkers 5(1), 9-23.
Abdel-Ghaffar F, Abdel-Gaber R, Bashtar AR, Morsy K, Mehlhorn H, Al Quraishy S, Saleh R. 2015. Hysterothylacium aduncum (Nematoda, Anisakidae) with a new host record from the common sole Solea solea (Soleidae) and its role as a biological indicator of pollution. Parasitology Research 114, 513-522.
Abollo E, Gestal C,
Pascual S. 2001. Anisakis infestation in marine fish and cephalopods from
Galician Waters: An Updated Perspective. Parasitology Research 87(6), 492-499.
Ak O, Kutlu S, Aydın İ.
2009. Length-weight relationship for 16 Fish Species from the Eastern Black
Sea, Türkiye. Turkish Journal of Fisheries andAquatic Sciences 9(1), 125-126.
Ali STA. 2021.
Prevalence of Parasites In Freshwater Fishes In The Southern Part Of Ligawasan
Marsh, Philippines. Procedia of Social Sciences and Humanities 1, 93-102.
Ali S, Akhter S,
Muhammad A, Khan, I, Khan WA, Iqbal MN, Umar S, Ahmed H, Ali Q. 2016.
Identification, Characterization and Antibiotic Sensitivity of Aeromonas
hydrophila, a causative agent of epizootic ulcerative syndrome in wild and
farmed fish from Potohar, Pakistan. Pakistan Journal Zoology 48(3), 899-901.
Anticamara JA, Go KT.
2016. Spatio-temporal declines in Philippine fisheries and its implications to
Coastal Municipal Fishers’ Catch and Income. Frontiers in Marine Science3. https://doi.org/10.3389/fmars.2016.00021.
Bachok Z, Mansor MI,
Noordin RM. 2004. Diet Composition and Food Habits of Demersal and Pelagic
Marine Fishes from Terengganu waters, East Coast of Peninsular Malaysia.
Bao M, Pierce GJ,
Strachan NJ, Pascual S, González-Muñoz M, Levsen A. 2019. Human Health,
Legislative and Socio-economic Issues Caused by the Fish-orne Zoonotic Parasite
Anisakis: Challenges in Risk Assessment. Trends in Food Science &
Technology 86, 298-310.
Barrett EJ, Liu Z,
Khamaisi M, King GL, Klein R, Klein BE, Casellini CM. 2017. Diabetic
Microvascular Disease: An Endocrine Society Scientific Statement. The
Journal of Clinical Endocrinology & Metabolism 102(12), 4343-4410.
BFARF GEFS. 2016.
Technical Working Group (TWG) Meeting BFAR-RFTC8 Conference Room, SSU-Mercede
Campus, Catbalogan City March 18, 2016. SEAFDEC.
Chai JY, Murrell KD,
Lymbery AJ. 2005. Fish-borne Parasitic Zoonosis: Status and Issues.
International Journal of Parasitology 35(11-12), 1233-1254. http://dx.doi.org/10.1016/j.ijpara.2005.07.013.
Chai JY. 2007.
Intestinal flukes. In: Murrell KD, Fried B. Food-Borne Parasitic Zoonoses: Fish
and Plant-Borne Parasites. New York: Springer 53-115. vol. 11. World Class
Parasites.
Cook HF. 2017. The
Protection and Conservation of Water Resources. Wiley Blackwell.
Cruz C, Barbosa C,
Saraiva A. 2007. Distribution of Larval Anisakids in Blue Whiting off
Portuguese Fish Market. Helminthologia 44, 21-24.
D’amico P, Malandra R,
Costanzo F, Castigliego L, Guidi A, Gianfaldoni D, Armani A. 2014.
Evolution of the Anisakis Risk Management in the European and Italian Context.
Food Research International 64, 348-362.
Dadar M, Alborzi A,
Peyghan R, Milad ADEL. 2016. Occurrence and Intensity of Anisakid Nematode
Larvae in Some Commercially Important Fish Species in Persian
Gulf. Iranian Journal of Parasitology 11(2), 239.
da Silva-Pinheiro, RH,
de Vasconcelos Melo FT, Monks S, Dos Santos JN, Giese EG. 2018. A New
Species of Procamallanus Baylis, 1923 (Nematoda, Camallanidae) from Astronotusocellatus
(Agassiz, 1831)(Perciformes, Cichlidae) in Brazil. ZooKeys 790, 21.
Dhole J, Jawale S,
Waghmare S, ChavanR. 2010. Survey of Helminth Parasites in Freshwater
Fishes from Marathwada region, MS, India. Journal of Fisheries and
Aquaculture 1(1), 1.
Diaz LS, Roa A, Garcia
CB, Acero A, Navas G. 2000. Length-Weight Relationships of Demersal
Fishes from the Upper Continental Slope off Colombia.
Duarte CM, Marbá N,
Holmer M. 2007. Ecology. Rapid Domestication of Marine Species.
Science 316(5823) 382-383. http://dx.doi.org/10.1126/science.1138042.
Edeh C, Solomon RJ. 2016.
Endoparasites of Oreochromis niloticus and Clarias gariepinusFound
in Utako Flowing Gutter. Direct Research Journal of Agricultural Food
Science 4(12), 361-373.
FAO. 2020. Food
Agriculture Organization. Aquaculture Department. 2013. Global Aquaculture
Production Statistics for the year.
Fierro P, Valdovinos C,
Arismendi I, Díaz G, De Gamboa MR, Arriagada L. 2019. Assessment of
Anthropogenic Threats to Chilean Mediterranean Freshwater Ecosystems:
Literature Review and Expert Opinions. Environmental Impact Assessment
Review 77, 114-121.
Fitriani EN, Rozi Arief
M, Suprapto H. 2019. Prevalence and Intensity of Ectoparasites in Gabus
fish (Channa striata) at Cangkringan Fishery Cultivation Technology
Development Center, Sleman, Yogyakarta. IOP Conference Series: Earth and
Environmental Science 236, 012095. https://doi.org/10.1088/1755-1315/236/1/012095
Frainer A, McKie BG,
Amundsen PA, Knudsen R, Lafferty KD. 2018. Parasitism and the
Biodiversity-Functioning Relationship. Trends in Ecology&Evolution 33(4), 260–268. https://doi.org/10.1016/j.tree.2018.01.011.
Gutierrez JP,
Agudelo-Botero M, Garcia-Saiso S, Zepeda-TenaC, Davila-Cervantes CA,
Gonzalez-Robledo MC, Lozano R. 2020. Advances and Challenges on the Path
Toward the SDGs: Subnational Inequalities in Mexico, 1990–2017. BMJ Global
Health 5(10), e002382.
Hila Bu SS, Leong TS. 1997.
Fish Parasite Communities in Tropical Reservoirs along Perak River, Malaysia.
Hydrobiologia 356, 175-181.
Iqbal MN, Ashraf A,
Iqbal I. 2019. Parasites of Fish: APotential Public Health Concern. PSM
Microbiology 4(2), 53-55.
Karl H. 2008.
Nematode Larvae in Fish on the German Market 20 years of Consumer-Related
Research. Archiv für lebensmittelhygiene 59(3), 107-116.
Khalil MI, El-Shahawy
IS, Abdelkader HS. 2014. Studies on Some Fish Parasites of Public Health
Importance in the Southern Area of Saudi Arabia. Revista Brasileira de
Parasitologia Veterinária 23, 435-442.
Khan RA. 2012.
Host-Parasite Interactions in Some Fish Species. Journal of Parasitology
Research1-7.
Kawel SM, Godspower RO,
Balarabe MR, Akaniru RI. 2016. Prevalence of Gastrointestinal Helminth
Parasites of Clarias gariepinius in Abuja, Nigeria. Sokoto. Journal
of Veterinary Sciences 14, 26-33.
Labrador K, Agmata A,
Palermo JD, Ravago-Gotanco R, Pante Ma J. 2021. Mitochondrial DNA Reveals
Genetically Structured Haplogroups of Bali Sardinella (Sardinellalemuru) in
Philippine Waters. Regional Studies in Marine Science 41, 101588. https://doi.org/10.1016/j.rsma.2020.101588.
Lafferty KD, Morris AK. 1996.
Altered Behavior of Parasitized Killifish Increases Susceptibility to Predation
by Bird Final Hosts. Ecology 77(5), 1390-1397.
Leela B, Rao KR. 2014.
Nematode Parasites in a Freshwater Fish Glossogobius giuris (Hamilton-Buchanan,
1822) at Lower Manair Dam, Karimnagar Dt. Andhra Pradesh, India. IOSR
Journal of Pharmacy and Biological Sciences 9(2), 37-40.
Levsen A, Lunestad BT,
Berland B. 2005. Low Detection Efficiency of Candling as a Commonly Recommended
Inspection Method for Nematode Larvae in the Flesh of Pelagic Fish. Journal of
Food Protection 68(4), 828-832.
Llarena-Reino M, Abollo
E, Regueira M, Rodríguez H, Pascual S. 2015. Horizon Scanning for Management of
Emerging Parasitic Infections in Fishery Products. Food Control 49, 49-58.
Lucas JS, Southgate PC,
Tucker CS. 2019. Aquaculture: Farming Aquatic Animals and Plants. Wiley
Blackwell. ISBN: 978-1-118-68793-2.
Margolis L, Esch GW,
Holmes JC, Kuris AM, Schad G. 1982. The Use of Ecological Terms in
Parasitology (Report of an Ad Hoc Committee of the American Society of
Parasitologists). The Journal of Parasitology 68(1), 131-133.
Miah G, Rafii MY,
Ismail MR, Puteh AB, Rahim HA, Asfaliza R, Latif MA. 2013. Blast
Resistance in Rice: AReview of Conventional Breeding to Molecular
Approaches. Molecular Biology Reports 40, 2369-2388.
Molnár K, Buchmann K,
Csaba S. 2006. Phylum Nematoda. In: PTK Woo, editors, Fish Diseases and
Disorders. Protozoan and Metazoan Infections. Oxfordshire: CABI Publishing. 2nd
edition 1, 417-443.
Moravec F, Van As LL. 2015.
Procamallanus (Procamallanus) spp. (Nematoda: Camallanidae) in Fishes of the
Okavango River, Botswana, including the description of P.pseudolaeviconchus n.
sp. parasitic in Clarias spp.(Clariidae) from Botswana and
Egypt. Systematic Parasitology 90, 137-149.
Nebel C, Harl J, Pajot
A, Weissenböck H, Amar A, Sumasgutner P. 2020. High prevalence and Genetic
Diversity of Haemoproteus columbae (Haemosporida: Haemoproteidae) in
feral pigeons Columba livia in Cape Town, South Africa. Parasitology
Research 119, 447-463.
Neves LR, Silva LMA,
Florentino AC, Tavares-Dias M. 2020. Distribution Patterns of Procamallanusinopinatus (Spirocamallanus)
(Nematoda: Camallanidae) and its Interactions with Freshwater Fish in
Brazil. Revista Brasileira de Parasitologia Veterinária 29.
Pietrock M, Hursky O. 2011.
Fish and Ecosystem Health as Determined by Parasite Communities of Lake
whitefish (Coregonus clupeaformis) from Saskatchewan Boreal :akes. Water
Quality Research Journal of Canada 46(3), 219-229.
Quiazon KMA. 2015.
Updates on Aquatic Parasites in Fisheries: Implications to Food Safety, Food
Security and Environmental Protection. Journal of Coastal Zone Management 18(396), 10-4172.
Rabadon MLL, Corpuz
MNC. 2021.Multivariate Analyses of Selected Hydro-Bacterial Variables
Along the Longitudinal Gradient of Orani River, Philippines. In IOP
Conference Series: Earth and Environmental Science 798(1), 012004.
IOP Publishing.
Ramos MH, Argarin THE,
Olaivar BA. 2020. Assessment on the Occurrence of Anisakid and other
Endoparasitic Nematodes Infecting Commercially-Important Fishes at Tayabas
Bay 27(2), 216-230. The Philippine Journal of Fisheries. https://doi.org/10.31398/tpjf/27.2.2020C0008
Ramos P. 2020.
Parasites in Fishery Products-Laboratorial and Educational Strategies to
Control. Experimental Parasitology 211, 107865.
Saeed R, Feng H, Wang
X, Zhang X, Fu Z. 2022. Fish Quality Evaluation by Sensor and Machine
Learning: A Mechanistic Review. Food Control 137, 108902. https://doi.org/10.1016/j.foodcont.2022.108902
Shalaby SIA, Ibrahim M,
Mahmoud NA, EL-Assely TM. 1989. Parasitological and Pathological Studies
on Encysted Metacercariae in the Musculature and Different Organs of Tilapia
nilotica. Egypt Journal Computational Clinical Pathology 2(1), 186-212.
Shamsan EF, Al-Jobory
HJ. 2018. Microbial Status of Sun-Dried Fish (Wazef) Sold in Different
Yemeni Markets. PSM BiologicalResearch 3(1), 1-8.
Shea-Donohue T, Qin B,
Smith A. 2017. Parasites, Nutrition, Immune Responses, and Biology of
Metabolic Tissues. Parasite Immunology 39(5), e12422.
Soares Magalhães RJ,
Salamat MS, Leonardo L, Gray DJ, Carabin H, Halton K, McManus D, Williams GM,
Rivera P, Saniel O, Hernandez L, Yakob L, McGarvey S, Clements A. 2014.
Geographical Distribution of Human Schistosoma japonicum infection in
the Philippines: Tools to Support Disease Control and Further Elimination.
International Journal for Parasitology 44(13), 977–984.
Tenorio JCB, Molina EC. 2021.
Monsters in our Food: Food-borne Trematodiasis in the Philippines and Beyond:
Veterinary Integrative Sciences 19(3), 467-485. https://doi.org/10.12982/VIS.
2021.038.
World Health
Organization – WHO. Control of Food-borne Trematode Infections. Geneva:
WHO; 1995. 107 WHO Technical Report Series 849.
Wu F, Ghamkhar R,
Ashton W, Hicks AL. 2019. Sustainable Seafood and Vegetable Production:
Aquaponics as a Potential Opportunity in Urban Areas. Integrated
Environmental Assessment and Management 15(6), 832-843.
Yooyen T, Wongsawad C,
Kumchoo K, Chaiyapo M. 2006. A New Record of Clinostomumphilippinensis (Valasquez,
1959) in Trichogaster microlepis (Gunther, 1861) from Bung Borapet,
Nakhon Sawan, Thailand. Southeast Asian Journal Tropical Medical Public
Health 37(3), 99-103.
Ziarati M, Zorriehzahra
MJ, Hassantabar F, Mehrabi Z, Dhawan M, Sharun K, Emran TB, Dhama K, Chaicumpa
W, Shamsi S. 2022. Zoonotic Diseases of Fish and Their Prevention and
Control. Veterinary Quarterly 42(1), 95–118. https://doi.org/10.1080/01652176.2022.2080298.










%20in%20full.JPG)


0 comments:
Post a Comment