Sasikala Shanmugam, Ramganesh Selvarajan, and Sundararaj Thangiah, from the different institute of the India. wrote a research article about, Staphylococcus aureus Drug Resistance in Sinusitis Patients. entitled, Drug resistance of Staphylococcus aureus in sinusitis patients. This research paper published by the International Journal of Biosciences (IJB). an open access scholarly research journal on Biosciences. under the affiliation of the International Network For Natural Sciences | NNSpub. an open access multidisciplinary research journal publisher.
Abstract
In this study on
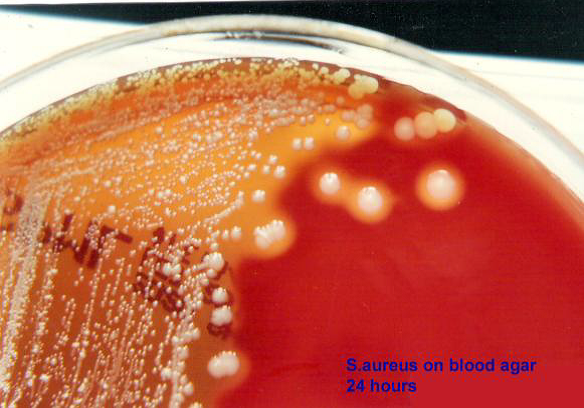
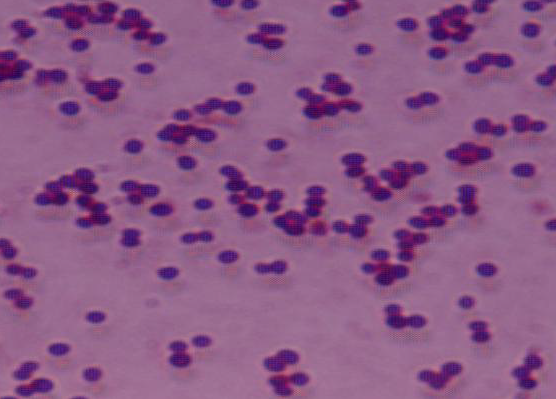
Sinusitis patients, we obtained 45 strains of Staphylococcus aureus. Antibiotic
pattern of Staphyloccus aureus showed that resistance to Quinolones
was 21% and 33% towards ciprofloxacin andoflaxacin respectively. Resistance to
cephalosporins was 50% to cefuroxime, 41% and 50% to cefaperazone and
cefotaxime respectively. Least resistance was noticed against aminoglycosides
viz. Amikacin 47% and Gentamicin 21%. Resistance to Ampicillin and amoxicillin
was 60% and 64% respectively. Oxacillin resistance was seen in 26% of the
strains. Of the 45 isolates, 6 were found to be resistant for oxacillin .
All these six isolates were subjected to Polymerase Chain Reaction (PCR) and
they possessed the mecA gene. Correlation existed between the presence
of mecA gene and oxacillin resistance in Staphylococcus aureus and
these strains can be considered as MRSA and the patients can be advised for vancomycin
therapy. Oxacillin resistance determination by phenotypic methods takes 24
hours to infer whereas PCR for mecA gene took only 6 hours. So the PCR
techniques for the detection of mecA gene can be considered as gold
standard (Rapid, Quick and accurate diagnosis) method for the detection of MRSA
in spite of the cost involved.
Read more : Assessing the Impact of Banana Plantation on Flora in Niagaramadougou, Côte d’Ivoire | InformativeBD
Introduction
Sinusitis is defined as
inflammation of one or more of the paranasal sinuses caused by bacterial or
viral infection; air-filled cavities in facial bones lined with pseudo
stratified ciliated columnar epithelium and mucous goblet cells (Nord et al.,
1995). There are several paired paranasal sinuses, including the frontal,
ethmoid, maxillary and sphenoid sinuses. Maxillary sinuses are located behind
the check bones and inflammation causes pain or pressure in the cheek
(maxillary) area. They are present at birth and continue to develop as long as
teeth erupt. Tooth roots in some cases, can penetrate the floor of these
sinuses. Frontal sinuses are located on both sides of the forehead and
inflammation causes pain or pressure in the frontal sinus cavity. These sinuses
are late in developing and so infection here is uncommon in children (Orobello
et al., 1991). Ethmoid sinuses are located between the eyes and inflammation
causes pain or pressure pain between eyes. They resemble a honeycomb and are
vulnerable to obstruction. Sphenoid sinuses are located behind the eye and
inflammation causes pain or pressure behind the eyes, but often refers to the
vertex of the head. They are usually present at the age of 3 and are fully
developed at the age of 12 (Nord et al., 1995).
The symptoms are generally the same in both acute and chronic rhinosinusitis. The symptoms include-nasal symptoms (facial congestion, facial pain-pressure fullness and headache), Oropharyngeal symptoms (halistosis, dental pain, cough and ear pain, pressure fullness) and, systematic symptoms (fever and fatigue). The symptoms in single or combine occur. Acute and chronic sinusitis may be accompanied by thick purulent nasal discharge (usually green in colour, with or without blood) and localized headache (toothache) are present and it is these symptoms that can differentiate sinus related (or rhinogenic) headache from other headache phenomena such as tension headache and migraine headache (Salord et al., 1990).
It is important to
diagnose nasal complaints accurately, because sinusitis requires antibiotics
for rapid resolution. Untreated sinusitis can lead to serious and possibly life
threatening complications. The clinical diagnosis of sinusitis is difficult
because of the overlap in the symptoms of rhinitis and sinusitis.
Several studies in adults have shown a good correlation between cultures of the middle meatus and the sinus aspirates in patients with acute sinusitis, especially when purulence is seen in the middle meatus (Walder et al., 1981). In many geographic areas, amoxicillin is a reasonable first-line antibiotic. Although trimethoprim- sulfamethoxazole and erythromycin- sulfisoxazole have traditionally been used as first line antibiotic for patients with acute bacterial sinusitis, surveillance studies indicate the development of significant pneumococcal resistance from alteration of penicillin binding proteins. Erythromycin alone provides unsatisfactory coverage and is effective against β -lactamase producing organisms. When first line agents have failed or there is a high prevalence of β–lactamase resistance, amoxicillin or clavulanate or second or third-generation cephalosporins (e.g., cefuroxime, cefpodoxime, cefprozil) provide broader coverage. First-generation cephalosoprins (eg-cephalexin) and second generation cephalosporins (eg, cefaclor) provide improved coverage. Several quinolones (eg, ciprofloxacin, gatifloxacin, levofloxacin, moxifloxacin) have specific indications for the treatment of sinusitis, but these should be reserved for second or third time use or for more serious infections.
MRSA stands for
methicillin resistant Staphylococcus aureus and also multi-resistant
Staphylococcus aureus. S. aureus strains which are resistant to the normal
antibiotics were successfully treated with Vancomycin (Mark et al., 2002). This
is one of the antibiotics used to treat emerging multi-resistant organisms. It
has evolved an ability to survive treatment with beta-lactamase resistant
beta-lactam antibiotics, including methicillin, dicloxacillin, nafcillin, and
oxacillin. MRSA is especially troublesome in hospital-associated (nosocomial)
infections. The methicillin resistance gene (mecA) encodes a methicillin
resistant penicillin-binding protein that is not present in susceptible strains
and is believed to have been acquired from a distantly related species. mecA is
carried on a mobile genetic element. Many MRSA isolates are multiply resistant
and are susceptible only to glycopeptide antibiotics such as Vancomycin and
other investigational drugs (Mark et al., 2002). MRSA isolates have decreased
susceptibility to glycopeptides. DNA fragments of mecA gene derived from MRSA
are used as a probe and this has been reported to be a means of identifying
methicillin resistance. More recently, several attempts to detect the presence
of the mecA gene by the Polymerase Chain Reaction (PCR) have also been reported
(Araj et al., 1991)
The widespread emergence of methicillin resistant Staphylococcus aureus (MRSA), especially in various types of nosocomial infections, is a serious clinical problem worldwide. The incidence of methicillin resistance among nosocomial isolates of S. aureus is higher than 70% in some Asian countries such as Taiwan, China, and Korea. Recently, MRSA has also emerged in the community setting in some countries, including Asian countries (Duong,D et al.,). One of the cardinal features of the rapid emergence of MRSA in many parts of the world is the dissemination of specific clones; this has contributed to the accelerated increases in the incidence of MRSA. Therefore, it is important to investigate the genotypic characteristics and evolutionary pathway of MRSA clones as well as the genetic relatedness of the strains isolated in different geographic regions.
The aim of the present
work is to evaluate the Antimocrobial activity of Staphylococcus aureus from
sinusitis patients with respect to different antibiotics and to detect the
Methicillin Resistant Staphylococcus aureus (MRSA) using genotypic method,
rather using a phenotypic method. So the PCR techniques for the detection of
mecA gene can be considered as gold standard (genotypic method). Accordingly,
we disclose that mecA gene carrying Staphylococcus aureus were considered as
MRSA and the patients who carry MRSA were advised to take Vancomycin therapy rather
going with other antibiotics.
Reference
Araj, Talhouk RS,
Simaan CJ, Maasad. 1991. Discrepancies between mecA gene PCR &
Conventional tests used for detection of Methicillin resistant Staphylococcus
aureus. International Journal of Antimicrobial Agent 11, 45-52.
Archer GL, Pennell E.
1990. Detection of methicilin resistance in Staphylococcus by using a DNA
probe. Antimicrobial Agents and chemotherapy 34, 1720-4.
Bect WD, Berger B,
Kayser FH. 1980. Additional DNA in methicillin resistant Staphylococcus
aureus and molecular cloning of mec-specific DNA. Bacteriol. 165, 373
– 378
Bogle, Bob RB, Graciela
S. 1999. Acquiring vancomycin resistant Staphylococcus aureus perspective
on measures needed for control. Ann. Intern. Med. 124(3), 329-34.
Boyce JM. 1990. Increasing
prevalence of Methicillin Resistant Staphylococcus aureus in the
United states. Infect control Hosp, Epidemiol. 11, 639-42
Nord. CE. 1995. The
role of anaerobic bacteria in recurrent episodes of sinusitis and tonsillitis
ClisInfect. Dis.20, 1512-1524.
Chikramane SG, Mathews
N, Stewart D. 1991. Tn 54 inserts in methicillin-resistant Staphylococcus
aureus from Australia and England. J. Gen. Microbial. 137, 1303-1311.
Clewell DB. 1998. Conjucative
transposons p.130-139. In F.j.de Bnijn, J.R. Lupski and G.M. Weinstock
(ed.), Bacterial Genomes, Chapman and Hall, New York, N.Y.
Lews S, Montgomery.
1983.Sphenoid Sinusitis. N. Eng. J. Med. 309, 1149-1154.
Doem GV, Bruggeman AB,
Pierce G, Holley HP, Rauch A. 1997. Antibiotic resistance among clinical
isolates of Haemophilus influenzae in the United states in 1994 and
1995 and detection of beta – lactamase positive strains resistant to
amoxicillin-clavanulate; results of a national mulicenter surveillance study
Antimicrob. Agents Chemother. 35, 1661-1665.
Dubin M, Childeramance
S. 1991. Physical mapping of mecA gene region of on American methicillin
resistance Staphylococcus aureus strains. Antimicrobial Agents
Chemother. 35, 1661-1665.
Duong G, Thompson.
2004. Methicillin resistance Staphylococcus aureus Antimicrob.
Agents Chemother. 37, 1552-57.
Finegold S.M. 1997.
Anaerobic bacteria in human disease .Orland’s, FL Academic press Inc.
Gold SM, Tami TA. 1997. Role
of middle meatus aspiration culture in the diagnosis of Chronic Sinusitis.
Laryyngoscope 107, 1586-1589.
Gordts F, AbuNassar I,
Clement PA, Pieard D, Kaufman KL. 1999. Bacteriology of the middle meatus
in children. Int. J. Pediatr. Otorhinolaryngol 48, 163-7.
Gwaltney J.J. 1996. Acute
community acquired sinusitis .Clinical infection of disease 23,1209-1212.
Hart SA, Phelps K,
Mulligan M.E, 1995. Instability and discriminatory power of
methicillin resistant Staphylococcus aureus typing by restriction
endonucleases analysis of plasmid DNA compared with those of other molecular
methods. J. Cl. Microbial. 33, 2022-6.
Hiramatsu K, Hanaki H,
Yubuta, Oguri T, Tenover FC. 1997. Methicillin- Resistant Staphylococcus
aureus clinical strains with reduced vancomycin susceptibility. J.
Antimicrobial Chemother. 40, 135-6.
Incaudo W. 1998. Diagnosis
and treatment of acute and subacute sinusitis in children and adults. Clin.
Rev. Allergy Immunol. 16, 157-204.
Jacobs MR, Bajaksouzian
Z, Pankuch A. 1999. Susceptibilities of Streptococcus pneumoniae and Haemophilus
influenzae to 10 oral Antimicrob agents based on pharmacodynamic
parameters, US surveillance study, Antimicrob. Agents Chemother. 460-465.
Kazuhisa M, Wakio M.
1991. Identification of Methicillin-Resistant Strains of Staphylococci by
Polymerase Chain Reaction. p. 2240-2244
Lacey RW. 1972. Genetic
control in Methicillin Resistant strains of Staphylococcus aureus. J. Med.
Microbial. 5, 197-508.
Lee CA. 1996. Pathogenicity
islands and the evolution of bacterial pathogens. Infect. Agents Dis. 5, 1-7.
Mark AR, Raynor R,
Edward JF, Hajo G. 2002. The evolutionary history of
methicillin-resistant Staphylococcus aureus (MRSA). PNAS 99
(11), 7687-7692.
Nadel L, Kennedy. 1999.
Endoscopically guided sinus cultures in normal subjects. J. Rhinol. 13,
37-90.
Orobello PR, Belcher.
1991. Microbiology of chronic Sinusitis in children. Arch. Otolaryngol.
Surg. 117, 980-983.
Paterson, David L.
2001. Reduced susceptibility of Staphylococcus aureus to
vancomycin – A review of current knowledge. Communicate Diseases intelligence 23.
Quintiliani R,
Courvalin. 1995. Mechanism of resistance to antimicrobial agents. In
P.R.Murray, E.J.Barom. M.A. Pfaller, F.C.Tenover, and R.H.Yolden (ed.,), Manual
of clinical Microbiology. American society for Microbiology, Washington.
Rechia GD, Hall. 1995. Gene
cassettes; a new class of mobile element. Microbiology 141, 3015-3027
Salord F, Gaussorgues,
Marti-Flich J. 1990. Noscomial Maxillary sinusitis during mechanical
ventilation- a prospective comparison of orotracheal versus the nosotracheal
route for incubation. Intensive Care Med. 18, 390-393.
Sjostrom JE, Lofdahi S,
Philipson. 1975. Transformation reveals a chromosomal locus of the genes
for the methicillin resistance of Staphylococcus aureus. J.
Bacteriol. 123, 905-913.
Spector SL, Bemstein
IL, Berger LIJT, Kaliner MA, Schuller DE. 1998. Parameters for the
diagnosis and management of sinusitis- complete guidelines and references. J.
Allergy Clin. Immunol. 102, 117-44.
Tos M. 1983. Distribution
of Mucous Producing elements in the respiratory tract. Differences between
upper and lower ainway. Eur J. Respir Dis. Suppl. 128, 296-79.
Unals, Wernerk,
Degirolami P, Barsantif, Ellopoyles G. 1994. Comparison of tests for the
detection of methicillin –resistant Staphylococcus aureus in a
clinical microbiology laboratory. Antimicrobial Agents Chemother. 38, 345-7.
Vogan, Bolger WE,
Keyes. 2000. As endoscopically guided Sinonal Cultures: a direct
comparison with maxillary sinus aspirates cultures. Otolaryngol Head Neck
Surg. 12, 370-373.
Walder, Mill GJ, Brown
AD, Ledema I, Salomon N, Bluestone CD. 1981. Acute maxillary sinusitis in
children. N. Engi J. Med. 304, 749-54.
Yauck JS, Smith TL.
2006. Patient undergoing sinus surgery constructing a demographic Profile. 116, 135-91.











%20in%20full.JPG)


0 comments:
Post a Comment