Md. Shariful
Islam, Mahbub Hasan Joy, and Aurnob
Sarker, from the different institute of Bangladesh. wrote a Research Article
about, Antimicrobial Resistance of Staphylococcus aureus in Frozen Chicken
Meat: A Prevalence Study. Entitled, Prevalence and antimicrobial resistance
pattern of Staphylococcus aureus from frozen chicken meat. This research paper
published by the International Journal of Biosciences (IJB). an open access
scholarly research journal on Biosciences. under the affiliation of
the International Network For Natural Sciences| INNSpub. an open
access multidisciplinary research journal publisher.
Abstract
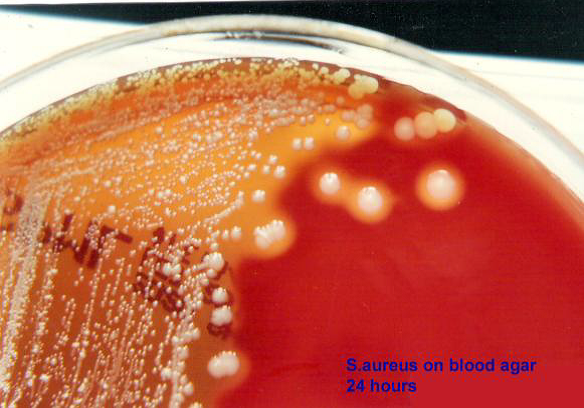
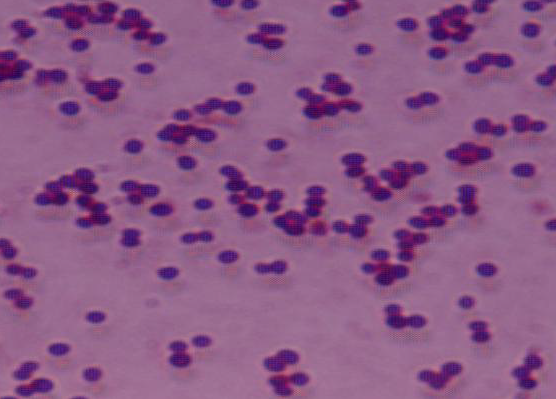
Staphylococcus aureus is a pathogenic bacterium known for its ability to cause infections in both humans and animals. A major concern is its rapid development of resistance to various antibiotics. Therefore, the present research aimed to screen S. aureus and analyze the antimicrobial resistance patterns of isolates obtained from frozen chicken meat samples collected from popular super shops in Sylhet metropolitan city, Bangladesh. S. aureus was identified through conventional culture and biochemical testing procedures from collected forty samples, while the cefoxitin disk diffusion technique was employed to detect methicillin-resistant S. aureus (MRSA). Among the samples, 65% were contaminated with S. aureus, with 42.31% of these isolates detected as MRSA. Notably, all MRSA isolates were found to be multidrug-resistant (MDR). Across all S. aureus isolates, resistance to methicillin was the highest (100%). High levels of resistance were noted against ampicillin (88.46%), nalidixic acid (84.62%), and azithromycin (65.38%). Conversely, all isolates showed 100% sensitivity to imipenem. The presence of multidrug-resistant S. aureus in chicken meat samples emphasizes the need of keeping good hygiene protocols by food handlers in super shops. Implementing these measures is vital to mitigating both the risk of MDR S. aureus contamination and spread.
Introduction
According to the World Health Organization (WHO), food-borne diseases are typically caused by bacteria present in food or water. One of the most common causes of these illnesses is Staphylococcus aureus (Scallan et al., 2011). It is considered as the thirdleading global cause of food-related diseases and an opportunistic pathogen in both humans and animals (Aydin et al., 2011). Naturally, S. aureus is widely distributed throughout the globe, but food is the main source of infection (Hennekinne, 2018). It grows best on a vast variety of regularly taken foods (Danbappa et al., 2018), but this varies from nation to nation because of regional differences in culinary practices. Many factors, such as faulty food preparation, inadequate cooking, and tainted water or raw ingredients used in food preparation, might contribute to outbreaks (Scallan et al., 2011).
Animal-derived meat serves as the main protein source, providing essential vitamins crucial for the growth, repair, and upkeep of body cells. This makes it indispensable for our daily functions in various regions across the globe (Pereira and Vicente, 2013; Olmedilla-Alonso et al., 2013). Among these, chicken meat, a widely consumed food globally, is often contaminated with antibiotic-resistant strains of S. aureus, posing a significant risk within the food chain (WHO, 2004). S. aureus and other pathogens contaminated meat by poor hygiene procedures used by slaughter personnel during meat processing, as well as other flawed abattoir procedures like improper evisceration of animals, which increases the risk of gut pathogens contaminating meat (Argudín et al., 2010; Leong et al., 2018).
The treatment options for food-borne illness caused by S. aureus are becoming narrowed due to the emergence of antimicrobial resistance (AMR) in pathogens, specifically methicillin-resistant S. aureus (MRSA) (Sallam et al., 2015). Recently, MRSA has shown multidrug-resistant (MDR) properties due to the improper use of antibiotics for treatment purposes, and as a result, infections are growing in humans (Wu et al., 2018). MRSA is recognized as one of the twelve microorganism families posing the most significant threat to public health (Wu et al., 2018). This threat is likely similar or higher in countries like ours. Consequently, the WHO has recently designated MRSA as "high priority 2 pathogen" (Okorie-Kanu et al., 2020). Unquestionably, antibiotics are the best way for treating infection caused by S. aureus (Leong et al., 2018). However, MRSA has developed resistance to all of the available betalactam antibiotics due to the presence of the mecA gene (Ito et al., 2012).
The AMR patterns and contamination of S. aureus in raw chicken meat collected from live bird market in Bangladesh is reported by many earlier studies (Akhi et al., 2019; Rahman et al., 2018; Datta et al., 2012). However, the processed and frozen meat is gaining popularities in cities like Sylhet, Bangladesh. Thus, it is imperative to assess the contamination status of processed chicken meat as well as frozen chicken, particularly with MRSA, in super shops.
Although there are few studies available in Bangladesh on MRSA presence in chicken meat from super shops (Parvin et al., 2021; Islam et al., 2019; Alam et al., 2015), a thorough investigation is needed to ensure the safety of frozen chicken meat in such super shops. Therefore, the study aimed at figuring out the prevalence of S. aureus and their multidrugresistant (MDR) patterns in frozen chicken meat from super shops within Sylhet metropolitan city, Bangladesh.
Reference
Abolghait SK, Fathi AG,
Youssef FM, Algammal AM. 2020. Methicillin-resistant Staphylococcus
aureus (MRSA) isolated from chicken meat and giblets often produces
staphylococcal enterotoxin B (SEB) in non-refrigerated raw chicken livers.
International Journal of Food Microbiology 328, 108669.
Akhi MA, Das NC, Banik
A, Abony M, Juthi M, Uddin ME. 2019. Detection of drug-resistant S.
aureus from poultry samples collected from different areas of Bangladesh.
Microbiology Research Journal International 29(1), 1-10.
Al Amin M, Hoque MN,
Siddiki AZ, Saha S, Kamal MM. 2020. Antimicrobial resistance situation in
animal health of Bangladesh. Veterinary World 13(12), 2713–2727.
https://doi.org/10.14202/vetworld.2020.2713-2727.
Alam ST, Howard
Meh-Buh, Fatema K, Haque KMF. 2015. Antibiogram of pre-processed raw
chicken meat from different supershops of Dhaka city, Bangladesh. Journal of
Allied Health Sciences 2(1&2), 45–52.
Ali Y, Islam MA,
Muzahid NH, Sikder MOF, Hossain MA, Marzan LW. 2017. Characterization,
prevalence, and antibiogram study of Staphylococcus aureus in
poultry. Asian Pacific Journal of Tropical Biomedicine 7(3), 253-256.
Argudín MÁ, Mendoza MC,
Rodicio MR. 2010. Food poisoning and Staphylococcus aureus enterotoxins.
Toxins (Basel) 2(7), 1751-1773. DOI: 10.3390/toxins2071751.
Aydin A, Sudagidan M,
Muratoglu K. 2011. Prevalence of staphylococcal enterotoxins, toxin genes,
and genetic relatedness of foodborne Staphylococcus aureus strains
isolated in the Marmara Region of Turkey. International Journal of Food
Microbiology 148(2), 99-106. DOI: 10.1016/j.ijfoodmicro.2011.05.007.
CLSI (Clinical and
Laboratory Standards Institute). 2023. Performance standards for
antimicrobial susceptibility testing. 33rd ed. CLSI supplement M100.
Danbappa AAR, Alhassan
KA, Shah MM. 2018. Isolation and identification of microbial contaminants
associated with commercial poultry feeds. Journal of Applied and Advanced
Research 3(5), 142-147.
Datta S, Akter A, Shah
I, Fatema K, Islam T, Bandyopadhyay A, Khan Z, Biswas D. 2012. Microbiological
quality assessment of raw meat and meat products, and antibiotic susceptibility
of isolated Staphylococcus aureus. Agriculture, Food and Analytical
Bacteriology 2(3), 187-194.
EN ISO 6888-1:1999/AMD
1. 2003. Microbiology of food and animal feeding stuffs: Horizontal method
for the enumeration of coagulase-positive staphylococci (Staphylococcus aureus and
other species); ISO: Geneva, Switzerland.
EUCAST. 2024.
European Committee on Antimicrobial Susceptibility Testing, Breakpoint tables
for interpretation of MICs and zone diameters. Version 14.0.
Guo Y, Song G, Sun M,
Wang J, Wang Y. 2020. Prevalence and therapies of antibiotic resistance
in Staphylococcus aureus. Frontiers in Cellular and Infection
Microbiology 10, 107.
Hennekinne JA. 2018. Staphylococcus
aureus as a leading cause of foodborne outbreaks worldwide. Academic
Press, 129-146. DOI: 10.1016/B978-0-12-809671-0.00007-3.
Islam MA, Parveen S,
Rahman M, Huq M, Nabi A, Khan ZUM, Ahmed N, Wagenaar JA. 2019. Occurrence
and characterization of methicillin-resistant Staphylococcus aureus in
processed raw foods and ready-to-eat foods in an urban setting of a developing
country. Frontiers in Microbiology 10, 503.
https://doi.org/10.3389/fmicb.2019.00503.
Ito T, Hiramatsu K,
Tomasz A, de Lencastre H, Perreten V, Holden MT, Coleman DC, Goering R, Giffard
PM, Skov RL, Zhang K, Westh H, O’Brien F, Tenover FC, Oliveira DC, Boyle-Vavra
S, Laurent F, Kearns AM, Kreiswirth B, Ko KS, Grundmann H, Sollid JE, John JF
Jr, Daum R, Soderquist B, Buist G; International Working Group on the
Classification of Staphylococcal Cassette Chromosome Elements (IWG-SCC). 2012.
Guidelines for reporting novel mecA gene homologues. Antimicrobial
Agents and Chemotherapy 56(10), 4997–4999.
https://doi.org/10.1128/AAC.01199-12.
Leong HN, Kurup A, Tan
MY, Kwa ALH, Liau KH, Wilcox MH. 2018. Management of complicated skin and
soft tissue infections with a special focus on the role of newer antibiotics.
Infection and Drug Resistance 11, 1959–1974.
https://doi.org/10.2147/IDR.S172366.
Masud AA, Rousham EK,
Islam MA, Alam MU, Rahman M, Mamun AA, Sarker S, Asaduzzaman M, Unicomb L. 2020.
Drivers of antibiotic use in poultry production in Bangladesh: Dependencies and
dynamics of a patron-client relationship. Frontiers in Veterinary Science 7,
78. https://doi.org/10.3389/fvets.2020.00078.
Okorie-Kanu OJ, Anyanwu
MU, Ezenduka EV, Mgbeahuruike AC, Thapaliya D, Gerbig G, Ugwuijem EE,
Okorie-Kanu CO, Agbowo P, Olorunleke S, Nwanta JA, Chah KF, Smith TC. 2020.
Molecular epidemiology, genetic diversity, and antimicrobial resistance
of Staphylococcus aureus isolated from chicken and pig carcasses, and
carcass handlers. PLoS One 15(5), e0232913. DOI:
10.1371/journal.pone.0232913.
Olmedilla-Alonso B,
Jiménez-Colmenero F, Sánchez-Muniz FJ. 2013. Development and assessment of
healthy properties of meat and meat products designed as functional foods. Meat
Science 95(4), 919-930.
Ou C, Shang D, Yang J,
Chen B, Chang J, Jin F, Shi C. 2020. Prevalence of
multidrug-resistant Staphylococcus aureus isolates with strong
biofilm formation ability among animal-based food in Shanghai. Food
Control 112, 107106.
Parvin MS, Ali MY,
Talukder S, Nahar A, Chowdhury EH, Rahman MT, Islam MT. 2021. Prevalence
and multidrug resistance pattern of methicillin-resistant Staphylococcus
aureus isolated from frozen chicken meat in Bangladesh.
Microorganisms 9(3), 636. DOI: 10.3390/microorganisms9030636.
Pereira PMDCC, Vicente
AFDRB. 2013. Meat nutritional composition and nutritive role in the human
diet. Meat Science 93(3), 586-592.
Peton V, Le Loir Y. 2014. Staphylococcus
aureus in veterinary medicine. Infection, Genetics and Evolution: Journal
of Molecular Epidemiology and Evolutionary Genetics in Infectious
Diseases 21, 602–615. https://doi.org/10.1016/j.meegid.2013.08.011.
Rahman MA, Rahman AKMA,
Islam MA, Alam MM. 2018. Multidrug-resistant Staphylococcus aureus isolated
from milk, chicken meat, beef, and egg in Bangladesh. Research in Agriculture
Livestock and Fisheries 5, 175–183.
Sallam KI, Abd-Elghany
SM, Elhadidy M, Tamura T. 2015. Molecular characterization and
antimicrobial resistance profile of methicillin-resistant Staphylococcus
aureus in retail chicken. Journal of Food Protection 78(10),
1879–1884. https://doi.org/10.4315/0362-028X.JFP-15-150.
Scallan E, Hoekstra RM,
Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. 2011.
Foodborne illness acquired in the United States–major pathogens. Emerging
Infectious Diseases 17(1), 7-15. DOI: 10.3201/eid1701.p11101.
Tang Y, Larsen J,
Kjeldgaard J, Andersen PS, Skov R, Ingmer H. 2017. Methicillin-resistant
and -susceptible Staphylococcus aureus from retail meat in Denmark.
International Journal of Food Microbiology 249, 72-76.
WHO. 2004.
Developing and maintaining food safety control systems for Africa: Current
status and prospects for change. In Proceedings of the Second FAO/WHO Global
Forum of Food Safety Regulators, Bangkok, Thailand. 12–14.
Wu S, Huang J, Wu Q,
Zhang J, Zhang F, Yang X, Wu H, Zeng H, Chen M, Ding Y, Wang J, Lei T, Zhang S,
Xue L. 2018. Staphylococcus aureus isolated from retail meat and
meat products in China: Incidence, antibiotic resistance, and genetic
diversity. Frontiers in Microbiology 9, 2767.
https://doi.org/10.3389/fmicb.2018.02767.
Zehra A, Gulzar M,
Singh R, Kaur S, Gill JPS. 2019. Prevalence, multidrug resistance, and
molecular typing of methicillin-resistant Staphylococcus aureus (MRSA)
in retail meat from Punjab, India. Journal of Global Antimicrobial
Resistance 16, 152–158.
Source : Prevalence and antimicrobial resistance pattern of Staphylococcus aureus from frozen chicken meat

















%20in%20full.JPG)

