Carolina D. Amper, from
the different institute of the Philippines. wrote a research article about, Bidens
pilosa Linn. Aqueous Extract against Postharvest Fungal Pathogens. entitled, In
vitro assay of Bidens pilosa Linn. aqueous extract against postharvest fungal
pathogens on Corn and Peanut. This research paper published by the International journal of Microbiology and Mycology (IJMM). an open access scholarly research
journal on Microbiology. under the affiliation of the International
Network For Natural Sciences | NNSpub. an open access
multidisciplinary research journal publisher.
Abstract
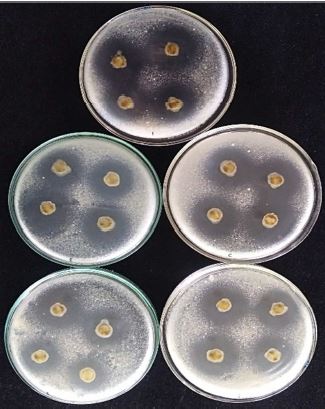
A bioassay was
conducted to assess the antifungal effects of the different concentrations
of Biden pilosa Linn. aqueous extract against fungal pathogens
isolated from corn and peanut seeds. The assay employed the disk diffusion
technique to determine the effects of the diffusible metabolites from B.
pilosa on the growth of the fungal species on potato dextrose agar (PDA).
The aqueous extract showed significant activity against Aspergillus
flavus, A. niger, Fusarium sp., and Penicillium sp. from corn
seeds. The best antifungal activity was observed in A. niger with
inhibitory zones as wide as 19.72mm in diameter. On the other hand, the fungal
isolates from peanut namely, A. flavus, A. niger, Penicillium sp.,
and Rhizopus stolonifer showed sensitivity to the aqueous extract
from B. pilosa. The best antifungal activity was recorded in Penicillium sp.
with the widest zone of inhibition of 24.87mm at 24 hours after incubation
(HAI). This in vitro study, therefore, confirms that the B.
pilosa aqueous extract inhibits the growth of fungal species associated
with corn and peanut seeds.
Read more : Trichoderma asperellum TR3 Viability Formulation: Packaging Variations | InformativeBD
Introduction
Corn and peanut seeds
are vulnerable to pathogenic fungal species before and after harvesting. Their
association with the stored seeds may eventually result in the deterioration of
seed quality. Although stored seeds apparently look healthy because of the
absence of physical damage, however, these may be contaminated with high levels
of mycotoxins produced by certain species of fungal pathogens. Mycotoxins are fungal
metabolites that cause grain quality deterioration, poor germination potential,
and reduced vigor. To prevent these problems, different control strategies
should be employed such as irradiation, chemical treatment, and biological
control. However, irradiation of seeds before storage is costly while the
application of chemical treatments poses hazards to humans and animals. With
these issues at hand, one of the promising options is the application of
botanical pesticides.
Several studies had
been conducted on the use of weed extract to control the growth of plant
pathogenic organisms. The water extracts from the weed species (A. conyzoides,
Oxalis corniculata, Phyllanthus debilis, Vernonia cinerea, and Desmodium
trifolium) were assayed for their antifungal activity against some plant
pathogenic fungi (Iqbal et al., 2001).
The extract from A.
conyzoides inhibited the mycelial growth of Rhizoctonia solani, Aspergillus
niger, and Phomopsis theae. In another study, the extract from O. corniculata
was active against A. niger while P. debilis suppressed the growth of P. theae.
The activity generally declined after three days of incubation, while A.
conyzoides remained active for nine days after incubation. Ethanolic extract of
Datura stramonium also contains significant antifungal potential against some
important plant pathogenic fungi and thus, could be used as an alternative to
chemical fungicides for the management of fungal infection in plants (Sharma et
al., 2014).
The antifungal
properties of B. pilosa were documented in some studies involving plant pathogenic
fungal species. Deba et al. (2007) first evaluated the antifungal potential of
this plant against Corticium rolfsii, Fusarium solani, and Fusarium oxysporum
using the hot water extracts from the roots, stem, and leaves. They found that
C. rolfsii was most suppressed as its growth was reduced almost all the tested
doses followed by F. oxysporum and F. solani. Extracts from stems and roots
exhibited greater fungicidal action than the extracts from the leaves. In
another experiment, the team also demonstrated the antifungal effects of the
essential oils and aqueous extracts from the flowers and leaves of B. pilosa
using the three fungal species. They again concluded that the extracts and oils
had antifungal activity on the fungal pathogens (Deba et al., 2008).
Polyacetylenes,
polyacetylene glycosides, flavonoids, flavone glycosides, aurones, chalcones,
okanin glycosides, phenolic acids, terpenes, pheophytins, fatty acids, and
phytosterols are among the chemical ingredients identified or isolated from the
various portions of B. pilosa (Xuan & Khanh, 2016). Many of these have been
identified as bioactive chemicals with pharmacological potential.
According to Silva et
al. (2011), as cited by Bartolome et al. (2013), 201 compounds have been
identified from this plant as compiled previously, comprising of 70 aliphatics,
60 flavonoids, 25 terpenoids, 19 phenylpropanoids, 13 aromatics, 8 porphyrins,
and 6 other compounds.
This study focused on
the assay of B. pilosa aqueous extract against common fungal pathogens
associated with corn and peanut seeds. The antifungal effect of the extract was
determined under in vitro conditions.
Reference
Ashafa OT, Afolaya AJ. 2009.
Screening the root extracts from Biden pilosa L. var. radiata (Asteraceae)
for antimicrobial potentials. Journal of Medicinal Plant Research 3(8), 568-572.
Bartolome AP,
Villaseñor IM, Yang W. 2013. Botanical properties, traditional uses,
phytochemistry, and pharmacology. Evidence-based Complementary and Alternative
Medicine 2013, 1-51.
Deba F, Xuan TD, Yasuda
M, Tawata S. 2008. Chemical composition and antioxidant, antibacterial,
and antifungal activities of the essential oils from Bidens pilosa Linn.
var. radiata. Food Control 19(4), 346-352.
Deba F, Xuan TD, Yasuda
M, Tawata S. 2007. Herbicidal and fungicidal activities and identification
of potential phytotoxins from Bidens pilosa L. var. radiata Scherff.
Weed Biology and Management 7(2), 77-83.
Iqbal CM, Meiyalaghan S, Wijesekara KB, Abeyratne KP. 2001.
Antifungal activity from water extracts of some common weeds. Pakistan
Journal of Biological Sciences 4, 843-845.
Sharma B, Srivatva KK,
Verma N, Niwas R, Singh M. 2014. Antifungal
potential of leaf extract of Datura stramonium L. against some
important plant pathogenic fungi. Acta Biologica Indica 3(2), 659-662.
Silva JJ, Cerdeira CD,
Chavasco JM, Cintra ABP, Silva CBP, Mendonca AN, Ishikawa T, Boriollo MFG,
Chavasco JK. 2014. In vitro screening antibacterial activity
of Bidens pilosa Linn. and Annona crassiflora Mart. against
oxacillin-resistant Staphylococcus aureus (ORSA) from the aerial
environment at the dental clinic. Rev. Inst. Med. Trop. Sao Paulo 56(4), 333-40.
Xuan TD, Khanh TD. 2016.
Chemistry and pharmacology of Bidens pilosa: an overview. Journal of
Pharmaceutical Investigation 46, 91-132.











%20in%20full.JPG)


0 comments:
Post a Comment