Md. Anwarul Kabir
Bhuiya, Md. Shahnawaz Parvez, Jahanara Nasrin, Md shoeb,
Md Abdur Rahman, Md. Asadul Islam, Md. Saiful Islam, and Samia Tabassum,
from the institute of Bangladesh. wrote a Research Article about, Green
Synthesis of Magnetite Nanoparticles from Calotropis gigantea for
Sunlight-Driven Dye Degradation. entitled, Biogenic Synthesis of Magnetite
Nanoparticles from Leaf and Latex Extract of Calotropis gigantea for Sunlight
Mediated Photocatalytic Degradation of MB Dye. This research paper published by
the International Journal of Biosciences (IJB). an open access scholarly
research journal on Biosciences. under the affiliation of the International
Network For Natural Sciences| INNSpub. an open access multidisciplinary
research journal publisher.
Abstract
Iron oxide nanoparticles, specifically magnetite (-NPs), have become widely used and a significant area of research due to their superparamagnetism and distinctive properties. As a result, scientists are diligently looking into new uses for these nanoparticles. The choice and use of synthesis techniques are important variables that can affect the size and characteristics of the nanoparticles (NPs). The use of toxic chemicals that are absorbed on the surface of the nanoparticles has been linked to a number of negative effects of chemical synthesis methods. The Green synthesis of nanoparticles has emerged as an eco-friendly method in response to environmental concerns, giving researchers the chance to worldwide investigate the potential of various herbs for nanoparticle synthesis. Green synthesis is considered as a novel, rapid, and eco-friendly method for obtaining metallic nanoparticles (NPs). In this study, magnetite nanoparticles (-NPs) were successfully synthesized using Calotropis gigantea (Akanda) leaf and latex extract. The NPs were identified and characterized by visual observation, Vibrating Sample Magnetometer (VSM), UV–vis spectrophotometry, Fourier Transform Infrared (FTIR) spectroscopy, Thermogravimetric Analysis (TGA) and Differential Thermal Analysis (DTA). The UV–vis spectrum showed board absorption without having any strong absorption peak, which confirmed the formation of -NPs. FTIR analysis showed the characteristic peak at 602 and 438, typical for Fe–O bond. The VSM curve doesn’t show any hysteresis loop which confirms the superparamagnetic behavior of -NPs. The saturation magnetization is 60emu/gm for CG leaf -NPs and 53emu/gm for CG latex -NPs. TGA confirms the high temperature stability of -NPs and the weight loss in TGA curve is due to the decomposition of organic biomolecules acting as a capping agent on the surface of the -NPs. The DTA curve shows an endothermic peak for the evaporation and decomposition of water and capping agents. The exothermic peak in DTA curve is due to the high temperature phase transition of -NPs to FeO. The photcatalytic activity of -NPs for the reduction of methylene blue (MB) dye was demonstrated by using UV–vis spectroscopy. It is expected that the synthesized -NPs could be a promising material to treat industrial wastewater via a profitable, sustainable, and eco-friendly approach.
Read more : Climate-Smart Agriculture Boosts Food Security Among Urban Gardeners in Réo, Burkina Faso |InformativeBD
Introduction
Industries like textiles, leather, plastic, foods, cosmetics, pharmaceuticals, and paper-pulp industries are increasing day by day. Bangladesh has the world's largest textile industry, and they release untreated effluents containing toxic chemicals into oceans, rivers, and canals. This poses a threat to marine life, ecosystems, and biodiversity, as well as to the health of people who rely on these water sources for daily living. The organic dyes present into the effluent of these industries are one of the most serious water pollutants. These dyes have a highly complex structure, due to which they are stable and pose detrimental effects on environment. So, the complete removal of organic dyes from industrial effluents before they are released into the environment (water bodies) is necessary. The degradation of dyes is one of the major challenges in wastewater treatment. Various methods like ozonation, adsorption, electrodialysis, flocculation, etc. are used for the removal of dye from wastewater. These methods are usually costly, slow, and less efficient (Pai et al., 2019).
Degradation of dyes with the help of metal or metal oxide NPs has been explored recently to develop a simple, cost-effective, and environmentally friendly method. Due to their unusual physical, surface chemical, and catalytic properties, metal/metal oxide NPs have attracted great attention (Jain et al., 2005). Low cost, good stability, easy synthesis, high magnetic permeability, and certain unique physiochemical properties make iron oxide NPs of great interest among the various metal oxide NPs. Fe3O4-NPs (magnetite NPs) have drawn a lot of attention among the various types of iron oxide nanoparticles, including FeO, α- Fe2O3, β- Fe2O3, and γ-Fe2O3, due to its notable qualities, including superparamagnetic property, biocompatibility, and high surface to volume ratio (Vinayagam et al., 2017). Due to their distinctive qualities, they have become indispensable in a variety of industries, including biomedicine, healthcare, agriculture and food, environmental remediation, energy, defense and aerospace, building and construction, automotive and textiles, and electronics (Dash et al., 2019). Iron oxide nanomaterials are effective at removing both organic and inorganic contaminants from water because of their exceptional properties (Qiu et al., 2011). The superparamagnetic behavior of magnetite nanoparticles (MNPs) is a benefit. The removal of the nanoparticles by the magnetic field after purification makes the purification procedure easier, more affordable, and secure to handle. These particles can be reused, thus making wastewater treatment more cost-effective and sustainable.
For the synthesis of Fe3O4 MNPs, a number of techniques have been reported in the literature, including the hydrothermal process Jiao (et al., 2008), sonochemical method Islam et al., (2011), micro-emulsion technique Deng, et al., (2003), electrochemical route Franger et al., (2004), sol-gel technique Unal et al., (2010) and co-precipitation method (Prasad et al., 2016).
However, the aforementioned methods are very expensive, call for expensive equipment, hazardous chemicals, and energy-intensive operating conditions. Additionally, the toxic byproducts produced by chemical methods pollute the environment (Vinayagam et al., 2017).
Increasing attention is being paid to biological
methods due to a number of drawbacks of chemical and physical methods. Here,
environmentally friendly, cost-effective green synthesis of Fe3O4 MNPs has been
developed as a substitute for traditional chemical and physical methods. Due to
its distinctive qualities, such as the use of plant or biological resources,
straightforward protocols, stable, improved NPs, and rapidness, green synthesis
is currently becoming a well-known and emerging technique (Dash et al., 2019).
Additionally, different phytochemicals found in plant extracts function during
the formation of NPs as capping and reducing agents. The rise in research
publications in recent years supports the growing acceptance of plantmediated
green synthesis of NPs (Vinayagam et al., 2017).
A survey of earlier literature suggests that extracts from various part of plants such as Carob extract, Carcia papaya hot water extract (Rahmani et al., 2020). Azadirachtaindica hot water extract (Jagathesan et al., 2018). Albiziaadianthifolia hot water extract (Taibet al., 2018). Platanusorientalis L. hot water extract (Sulaiman et al., 2018). Andean blackberry hot water extract (Nurbas et al., 2017). Shanghai white tea (Camellia sinensis) (Kumar et al., 2016). Amaranthusspinosus water extract (Shojaee et al., 2016). Hordeumvulgare aqueous extract (Muthukumaret al., 2015. Carob hot water extract (Awwad et al., 2012). Sargassummuticum (Seaweed) (Mahdavi et al., 2013). Plantain peel extract (Venkateswarluet al., 2013). Eucalyptus Globulus (Balamurugan et al., 2014). Ocimumsanctum (DaturaInoxiaDas et al., 2014). Centellaasiatica (Lakshmi et al., 2015). Jatropha gosspifolia Karkuzhali et al., 2015). Kappaphycusalvarezi (Seaweed) Yew et al., (2016), etc. have been explored for the synthesis of Fe3O4-NPs.
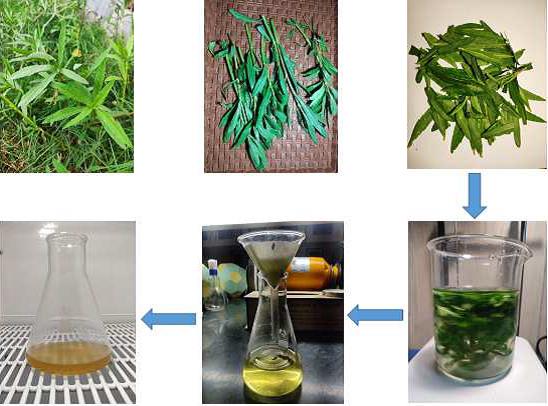
The green synthesis of Fe3O4-NPs using the leaf and latex extract of Calotropis gigantea has still not been done. Calotropis gigantea is abundantly found in various Asian countries and locally known as “Akanda” in Bangladesh. The plant was used to treat fevers, elephantiasis, nausea, vomiting, and diarrhoea as well as skin, digestive, respiratory, circulatory, and neurological disorders. Calotropis giganta's latex has been used as a treatment for cancer, arthritis, and as a snake bite remedy. Numerous plant parts are known to contain substances that serve as capping, reducing, and stabilising agents, such as alkaloids, carotenoids, flavonoids, starch, carbohydrates, essential oils, leguminvicilin, legumelin, vitamin C, oleoresin, gum resin, tannins, terpenes, and phenols (Ramesh et al., 2018).
Here, we
investigated for the first time how an extract from the leaf and latex of
Calotropis gigantea was used as a capping and reducing agent for the synthesis
of Fe3O4-NPs. Therefore, the primary goals of this study were to (a)
synthesizeFe3O4-NPs using the leaf and latex extract from Calotropis gigantea,
and (b) characterize Fe3O4-NPs using a variety of spectroscopic methods.
UV-visible spectroscopy has been used to investigate the photocatalytic
abilities of MNPs created by using Calotropis gigantea leaf and latex extracts
to break down the Methylene blue (MB) dye in aqueous solutions in the presence
of H2O2. When exposed to sunlight, the degradation reaction is
photocatalytically accelerated.
Reference
Pai Shraddha, 2019.
“Photocatalytic zinc oxide nanoparticles synthesis using Peltophorumpterocarpum leaf
extract and their characterization.” Optik 185, 248-255.
Jain AK. 2005.”Anion
recognition through novel C- thiophenecalix [4] resorcinarene: PVC based sensor
for chromate ions.” Talanta 65(3), 716-721.
Vinayagam, Ramesh,
Thivaharan Varadavenkatesan, Raja Selvaraj. 2017. “Evaluation of the
anticoagulant and catalytic activities of the Brideliaretusa fruit
extract-functionalized silver nanoparticles.” Journal of Cluster
Science 28, 2919-2932.
Dash, Asiman, Mohammed
Tameem Ahmed, Raja Selvaraj. 2019. “Mesoporous magnetite nanoparticles
synthesis using the Peltophorumpterocarpumpod extract, their antibacterial
efficacy against pathogens and ability to remove a pollutant dye.” Journal
of Molecular Structure 1178, 268-273.
Qiu, Guohong, 2011.
“Microwave-assisted hydrothermal synthesis of nanosized α-Fe2O3 for catalysts
and adsorbents.” The Journal of Physical Chemistry C 115, 4019626-19631.
Jiao, Hua, HeQing Yang. 2008.
“Controlled synthesis and magnetic properties of Fe3O4 walnut spherical
particles and octahedral microcrystals.” Science in China Series E:
Technological Sciences 51(11), 1911-1920.
Islam, Md Nazrul,
Jong-RyulJeong, Cheol Gi Kim. 2011. “A facile route to sonochemical
synthesis of magnetic iron oxide (Fe3O4) nanoparticles.” Thin Solid
Films 519(23), 8277-8279.
Deng Y. 2003.
“Preparation of magnetic polymeric particles via inverse microemulsion
polymerization process.” Journal of Magnetism and Magnetic Materials 257(1), 69-78.
Franger S, Berthet RP,
Berthon J. 2004. “Electrochemical synthesis of Fe3O4 nanoparticles in
alkaline aqueous solutions containing complexing agents.” Journal of Solid
State Electrochemistry 8, 218-223.
Unal B. 2010.”Synthesis,
conductivity and dielectric characterization of salicylic acid–Fe3O4
nanocomposite.” Materials Chemistry and Physics 123(1), 184-190.
Prasad Ch, Gangadhara
S, Venkateswarlu P. 2016. “Bio-inspired green synthesis of Fe3O4 magnetic
nanoparticles using watermelon rinds and their catalytic
activity.” Applied Nano science 6, 797-802.
Hernández-Hernández,
Aldahir A. 2020. “Iron oxide nanoparticles: synthesis, functionalization,
and applications in diagnosis and treatment of cancer.” Chemical Papers 74(11), 3809-3824.
Rahmani, Reyhaneh. 2020.
“Plant-mediated synthesis of super paramagnetic iron oxide nanoparticles
(SPIONs) using aloe vera and flaxseed extracts and evaluation of their cellular
toxicities.” Ceramics International 46(3), 3051-3058.
Jagathesan G, Rajiv P. 2018
“Biosynthesis and characterization of iron oxide nanoparticles using Eichhorniacrassipes leaf
extract and assessing their antibacterial activity.” Biocatalysis and
agricultural biotechnology 13, 90-94.
TaibIzza N. 2018.
“Green synthesis of iron oxide nanoparticles (Fe3O4-NPs) using Azadirachtaindica aqueous
leaf extract.” International Journal of Engineering and Technology 7, 4-18.
Sulaiman, Ghassan M,
AmerTawfeeq T, AmalNaji S. 2018. “Biosynthesis, characterization of
magnetic iron oxide nanoparticles and evaluations of the cytotoxicity and DNA
damage of human breast carcinoma cell lines.” Artificial cells,
nanomedicine, and biotechnology 46(6), 1215-1229.
Nurbas, Macid, Hamed
Ghorbanpoor, Hüseyin AVCI 2017. “An Eco-Friendly Approach to
Synthesis and Characterization of Magnetite (Fe3O4) Nanoparticles Using PlatanusOrientalisl.
Leaf Extract.” Digest Journal of Nanomaterials & Biostructures
(DJNB) 12(4).
Kumar, Brajesh. 2016.
“Phytosynthesis and photocatalytic activity of magnetite (Fe3O4) nanoparticles
using the Andean blackberry leaf.” Materials Chemistry and
Physics 179, 310-315.
Shojaee S, Mahdavi
Shahri M. 2016. “Green synthesis and characterization of iron oxide
magnetic nanoparticles using Shanghai white tea (Cameliasinensis) aqueous
extract.” Journal of Chemical and Pharmaceutical Research 8(5), 138-143.
Muthukumar, Harshiny,
Manickam Matheswaran. 2015. “Amaranthusspinosus leaf extract mediated FeO
nanoparticles: physicochemical traits, photocatalytic and antioxidant
activity.” ACS Sustainable Chemistry & Engineering 3(12), 3149-3156.
Awwad, Akl M, Nidá
Salem M. 2012 “A green and facile approach for synthesis of magnetite
nanoparticles.” Nanoscience and Nanotechnology 2(6), 208-213.
Mahdavi, Mahnaz. 2013.
“Green biosynthesis and characterization of magnetic iron oxide (Fe3O4)
nanoparticles using seaweed (Sargassummuticum) aqueous extract.” Molecules 18(5), 5954-5964.
Venkateswarlu, Sada. 2013.
“Biogenic synthesis of Fe3O4 magnetic nanoparticles using plantain peel
extract.” Materials Letters 100, 241-244.
Balamurugan,
Matheswaran, Shanmugam Saravanan, Tetsuo Soga. 2014. “Synthesis of iron
oxide nanoparticles by using Eucalyptus globulus plant
extract.” e-Journal of Surface Science and Nanotechnology 12, 363-367.
Das, Amlan Kumar,
AvinashMarwal, Ruchi Verma. 2014. “Daturainoxia leaf extract mediated
one step green synthesis and characterization of magnetite (Fe3O4)
nanoparticles.” Research and Reviews: Journal of Pharmaceutics and
Nanotechnology 2(2), 21-24.
Lakshmi PP, Mohan GK,
Rao KV. 2015. “Green synthesis & characterization of iron oxide
magnetic nanoparticles using Centellaasiatica plant—A theranostic
agent.” International Journal of Engineering Research &
Technology 3, 52-59.
Karkuzhali, Yogamoorthi
A, Yogamoorthi A. “Biosynthesis of iron oxide nanoparticles using aqueous
extract of Jatropha gosspifolia as source of reducing
agent.” International Journal of Nano Science and Nanotechnology 6(1), 47-55.
Yew, Yen Pin. 2016.
“Green synthesis of magnetite (Fe3O4) nanoparticles using seaweed (Kappaphycusalvarezii)
extract.” Nanoscale research letters 11, 1-7.
Ramesh AV. 2018.
“Facile green synthesis of Fe3O4 nanoparticles using aqueous leaf extract
of Zanthoxylumarmatum DC. For efficient adsorption of methylene
blue.” Journal of Asian Ceramic Societies 6(2), 145-155.
de JesúsRuíz-Baltazar,
Álvaro. 2019. “Eco-friendly synthesis of Fe3O4 nanoparticles:
evaluation of their catalytic activity in methylene blue degradation by kinetic
adsorption models.” Results in Physics 12, 989-995.
Prasad, Cheera,
GuthaYuvaraja, Ponneri Venkateswarlu. 2017. “Biogenic synthesis of Fe3O4 magnetic
nanoparticles using Pisumsativum peels extract and its effect on magnetic and
Methyl orange dye degradation studies.” Journal of Magnetism and Magnetic
Materials 424, 376-381.
Dhar, Palash Kumar. 2021.
“Green synthesis of magnetite nanoparticles using Lathyrussativuspeel
extract and evaluation of their catalytic activity.” Cleaner Engineering and
Technology 3, 100117.
Ahmad, Sharif. 2009.
“Soft template synthesis of super paramagnetic Fe3O4nanoparticles a novel
technique.” Journal of Inorganic and Organometallic Polymers and
Materials 19, 355-360.
Bishnoi, Shahana, Aarti
Kumar, Raja Selvaraj. 2018 “Facile synthesis of magnetic iron oxide
nanoparticles using inedible Cynometraramiflora fruit extract waste
and their photocatalytic degradation of methylene blue dye.” Materials
Research Bulletin97, 121-127.
Wang Kun. 2009
“Photocatalytic degradation of methylene blue on magnetically separable
FePc/Fe3O4 nanocomposite under visible irradiation.” Pure and Applied
Chemistry 81(12), 2327-2335.
Sirdeshpande, Karthikey
Devadatta. 2018. “Structural characterization of mesoporous magnetite
nanoparticles synthesized using the leaf extract of Calliandrahaematocephala and
their photocatalytic degradation of malachite green dye.” Applied
Nanoscience 8, 675-683.
Ahmad, Sharif.
2009. “Soft template synthesis of super paramagnetic Fe3O4nanoparticles a novel
technique.” Journal of Inorganic and Organometallic Polymers and
Materials 19, 355-360.
Das Chanchal. 2020.
“Green synthesis, characterization and application of natural product coated magnetite
nanoparticles for wastewater treatment.” Nanomaterials 10(8), 1615.
Shahwan Talal, 2011.
“Green synthesis of iron nanoparticles and their application as a Fenton-like
catalyst for the degradation of aqueous cationic and anionic
dyes.” Chemical Engineering Journal 172(1), 258-266.
Mohamed, Nadia Hussein. 2014.
“Antimicrobial activity of latex silver nanoparticles using Calotropis
procera.” Asian Pacific Journal of Tropical Biomedicine 4(11), 876-883.
Hassan, Mohammad HA. 2017.
“Phytochemical and antimicrobial of latex serum of Calotropis procera and
its silver nanoparticles against some reference pathogenic strains.” J
Ecol Health Environ 5(3), 65-75.
Gawade VV. 2017.
“Green synthesis of ZnO nanoparticles by using Calotropis procera leaves
for the photodegradation of methyl orange.” Journal of Materials Science:
Materials in Electronics 28, 14033-14039.
Das, Ratul Kumar. 2012.
“Microwave-mediated rapid synthesis of gold nanoparticles Using Calotropis
procera latex and study of optical properties.” International
Scholarly Research Notices.
Harne, Shrikant. 2012.
“Novel route for rapid biosynthesis of copper nanoparticles using aqueous
extract of Calotropis procera L. latex and their cytotoxicity on
tumor cells.” Colloids and Surfaces B: Biointerfaces 95, 284-288.
Chandhru M. 2022.
“Green synthesis of silver nanoparticles from plant latex and their
antibacterial and photocatalytic studies.” Environmental Technology 43(20), 3064-3074.
Rajendrachari,
Shashanka. 2020. “A fast and robust approach for the green synthesis of
spherical Magnetite (Fe3O4) nanoparticles by Tilia Tomentosa (Ihlamur) leaves
and its antibacterial studies.”
Bhuiyan, Md Shakhawat
Hossen. 2020. “Green synthesis of iron oxide nanoparticle using Carica
papaya leaf extract: application for photocatalytic degradation of remazol
yellow RR dye and antibacterial activity.” Heliyon 6(8)
Jalil WBF. 2017.”Low
toxicity superparamagnetic magnetite nanoparticles: One-pot facile green
synthesis for biological applications.” Materials Science and Engineering:
C 78, 457-4.
















%20in%20full.JPG)

