Seyede Negin Mirghasemi, Mina neginfar, Salar Jamali , Mina Allamoradi and Amaneh Hosseinikhah Choshali from the different institute of the Iran, wrote a research article about, Plant-Parasitic Nematodes in Peanut Fields, entitled, "Reported some species of plant parasitic nematodes from rhizosphere of peanut (Arachis ypogaea) fields". This research paper published by the International Journal of Mycrobiology and Mycology | IJMM. an open access scholarly research journal on Mycrobiology, under the affiliation of the International Network For Natural Sciences | INNSpub. an open access multidisciplinary research journal publisher.
Abstract
In order to identify of
peanut fields plants parasitic nematodes, 130 samples of soil around the roots
of peanut plants were collected in province of Guilan, during the summer and
fall of 2011. After extraction, killing, fixation and transferring to anhydrous
glycerol, the nematodes were mounted on permanent microscopic slides and
nematodes species identified by using light microscope, equipped with digital
camera, based on morphological and morphometric characters using valid keys. In
this study 20 species belonging 17 genera were identified, that are as
followes: 1- Aphelenchoides sacchari 2-Aphelenchus avenae 3- Basiria
graminophila 4-Coslenchus costatus 5-Ditylenchus myceliophagus 6-Filenchus
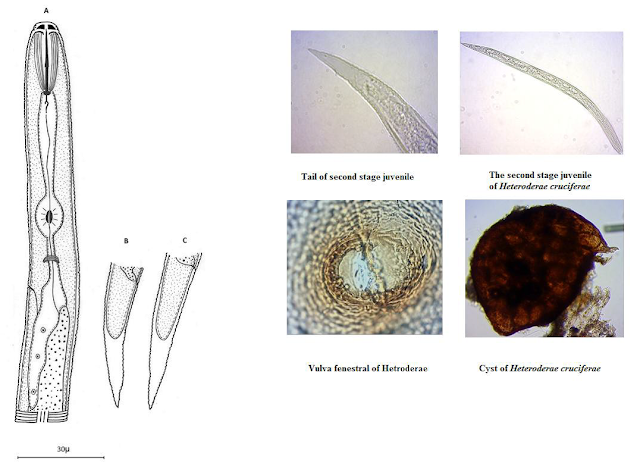
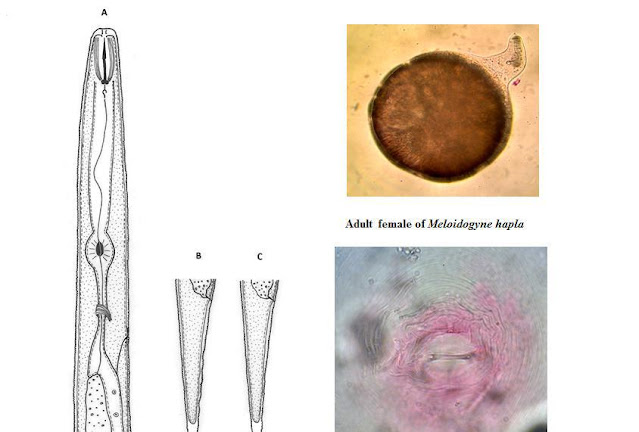
vulgaris 7-Helicotylenchus digonichus 8-Heterodera cruciferae 9-Meloidogyne
incognita 10-Meloidogyne hapla 11-Merlinius bavaricus 12-
Mesocriconemarusticum13- Mesocriconema curvatum 14-Paratylenchus nanus
15-Pratylenchus neglectus 16- Psilenchus hilarulus 17-Quinsulcius capitatus
18-Tylenchorhynchus annulatus 19- Tylenchorhynchus mashhoodi 20- Tylenchus
davainei. In thisstudy, 20 species belonging 17 genera were identified
that before just 6 Species2-5-7-11-15-16were reported from rhizosphere of Peanut in Iran. Other species (14 Species) are going to report from rhizosphere
of Peanut in Iran as a first.
Read more : Caladium bicolor: In Vitro Regeneration Insights | InformativeBD
Introduction
Arachis hypogaea, the peanut or groundnut, is an annual herbaceous plant in the Fabaceae (legume or bean family), with high protein content, vitamins and useful mineral compounds. Total commercial production of peanuts worldwide was 37.6 million tons, harvested from 24.1 million hectares, in 2010. Iran cultivated area The most under cultivating area is located in Guilan province of Iran as 90 present of peanut crop were cultivated in this area (Anon,2012).
Peanut Arachis hypogaea is considered one of the important food crops, produced in many subtropical and tropical countries; also it is a high value cash crop for small and large growers alike. It was listed as one of the twenty crop plants that stand between man and starvation (Wittwer, 1981). Numerous nematode species can damage peanut.(Minton &Baujard, 1990).Several plant parasitic nematodes were reported from peanut fields in South Eastern States of USA such as Belolaimus longicaudatus, Meloidogyne hapla, Meloidogyne arenaria, Mesocriconema ornatum and Pratylenchus brachyurus (Barker, 1992). Peanut root–knot nematode Meloidogyne arenaria is considered one of the most serious nematode pathogens of peanut in many parts of the world, attacking peanut roots, pegs and pods and causing substantial yield losses in severely infested fields (Minton & Baujard, 1990). Meloidogyne arenaria and M. hapla are the most important peanut crop loss agent in USA, which their populations have reached as economically damaging threshold (Hirunsalee et al.,1995). Elekcioglu et al. (1994) also have reported many species Aphelenchus avenae, Ditylenchus myceliophagus, D. valveus, Pratylenchus thornei, Tylenchorhynchus goffartri from peanut fields in the east mediterranean region of Turkey.
In order to improve cultivating steps in peanut and prevent the decline of peanut crop production in traditional cultures, the need to identify plant pathogens, is the first and most important step in disease management. Since the yield loss associated with plant pathogens including plant parasitic nematodes, this study aims to investigate the peanut nematodes.
Reference
Barker KR. 1992.
Effects of Meloidogyne hapla and Meloidogyne arenaria on
black rot severity in new Cylindrodadium-resistant peanut genotypes. Plant
Disease, 76, 352-357.
Brzeski WM. 1998. Nematodes
of Tylenchina in Poland and temperate Europe. Muzem I Inst. Zool.
PolskaAkad.Nauk.Warsaw, Poland, 396 P.
Brzeski MW, Dolinski
CM. 1998. Compendium of the genus Tylenchorhynchus Cobb, 1913
sensulato (Nematoda: Belonolaimidae). Russian Journal of Nematology6:189-199.
(Synonymy of six genera with Tylenchorhynchus).
Coolen WA, D´herde CJ. 1972.
A method for the quantitative exttissue. Ghent: State Nematology and Entomology
Research Station, 77p.raction of nematodes from plant.
De Grisse A. 1969.
Redescription ou modification de quelques dans letude des nematodes
Phytoparasit. Mededelingen Rijksfaculteit der landbouwwetenschappen Gent, 34,
351-369.
Elekcioglu˙IH,
Osnesorge B, Lung G, Uygun N. 1994. Plant parasitic nematodes in the East
Mediterranean Region of Turkey. Nematol. Medit. 22, 59-63.
Geraert E. 2010.
The Criconematidae of the world, Identification of the family Criconematidae (Nematoda).
Academia Press, Gent, Belgium.pp. 615.
Handoo ZA. 2000. A
Key and Diagnostic Compendium to the Species of the Genus Tylenchorhynchus
Cobb, 1913(Nematoda: Belonolaimidae). Journal of Nematology, 32(1), 20-34.
Handoo Z, Skantar AM,
Carta LK, Schmitt DP. 2005. Morphological and Molecular Evaluation
of MeloidogynehaplaPopulation Damaging Coffee (Coffee arabica) in Maui,
Hawaii.Journal of Nematology, 37(2), 136-145.
Hirunsalee A, Barker
KR, Beute MK. 1995. Infection, Reproduction Potential, and Root Galling by
Root-Knot Nematode Species and Concomitant Population on Peanut and
Tobacco.Jurnal of Nematology, 27(2), 172-177.
Jepson S. 1987.Identification
of root-knot nematodes (Meloidogynespecies). London, UK. C.A.B International,
293p.
Minton NA, Bajuard P. 1990.
Nematode parasite of peanut.Pp: 285-320. In: Plant Parasitic Nematodes in
Subtropical and Tropical Aagriculture (M. Luc, R.A. Sikora& J. Bridge,
eds). CAB.International Inst. Parasitology.Wallingford Oxon. UK.
Raski DJ. 1957.
Revision of the Genus Paraty lenchus Micoletzky, 1922, and describtions of new
species. Part 2 of Three parts. J. Nematol.7, 274-295.
Wittwer SH. 1981.
The 20 corps that stand between man and starvation. Farm chemicals, 144, 17-28.
Wouts WM. 2006.
Fauna New Zealand Kote Atitanaga Pepeke o Aotearoa Criconematina (Nematoda:
Tylenchida).Lincoln, Canterbury, New Zealand, 55, 232pp.
anonymous.2011.(http://www.iran.ir/about/city
/gilan. 5/12/2012).
anonymous.2009.Ebtekarnews.(http://www.ebt
ekarnews.com/ebtekar/news.aspx?nid=58380.5/ 12/2012).
Anonymous. 2000-2012.
Soya tech, growing opportunities. (http://www.soyatech.com/peanut_facts.htm.5/1
2/2012).
Source: Reported some species of plant parasitic nematodes from rhizosphere of peanut (Arachis ypogaea) fields




























%20in%20full.JPG)

