EA. Mahmoud, FH. Nada,
AA. Omar, GM. Hassan from the different institute of the Egypt.
wrote a research article about, Cellulase Overproduction: Trichoderma
harzianum Mutants . entitled, "Induction and characterization of
a cellulase overproducing mutant strains of Trichoderma harzianum". This
research paper published by the International Journal of Mycrobiology and Mycology | IJMM. an open access scholarly research journal on Mycrobiology
under the affiliation of the International Network For Natural Sciences |
INNSpub. an open access multidisciplinary research journal publisher.
Abstract
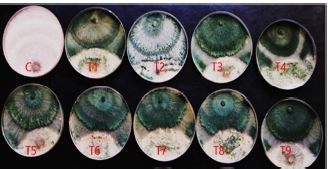
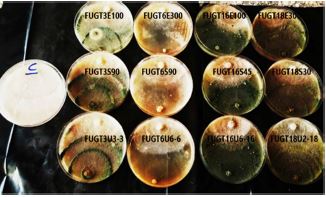
Nine Trichoderma isolates were isolated from different agricultural soil samples. All the Nine Trichoderma isolatesshowed positive test for cellulase as revealed by the formation of a clear zone on the screening medium containing Congo red as indicator. The percentage inhibitory effect of all the Nine Trichoderma isolates against growth of R. solani was calculated and ranged from 58 % to 64.9%. The qualitative and antagonistic results showed the isolate FUGT4 had the highest efficiency in antagonistic against R. solani. Molecular phylogenetic analysis was performed based on nucleotide sequences. The results indicate that the isolate FUGT4 are closely related to Trichoderma harzianum, with accession number OL953189. The selected strain was subjected to mutation using UV-radiation and 12 mutants were obtained when exposed to different time periods of radiation.These mutants were tested for their cellulase productivity compared to their wild type and the results showed that, the mutants were produced cellulase 0.921 IU/mL cellulase activities. The ISSR analysis of genomic DNA was performed to detect genetic diversity of these mutants with the wild type by using 6 primers. The mutants and their wild counterparts were tested for their antagonistic potently against to R. solani. The results showed that all mutants induced from the wild type Trichoderma were significantly better than the wild type when tested against R. solani.
Read more : Coccinia Grandis:Antiangiogenic Activity in Duck Embryos | InformativeBD
Introduction
Many microorganisms that produce various cellulolytic enzymes have been studied for several decades. The genus of Trichoderma has been especially famous for producing cellulolytic enzymes with relatively high enzymatic activity. Economic analyses have indicated that the production of cellulase is still a cost factor. It is therefore imperative to improve the production of cellulase in order to make the process more economically viable. Hence many traditional mutagenesis strategies have been applied to improve the production of cellulase. This technique is simple and successful many times as compare to the complication exists (Chand et al., 2005).
One of the alternative methods to control pathogens is to use biological control agents instead of chemical pesticides. Biological control is a safe and suitable method for humans and the environment. Trichoderma is one of the most suitable biocontrol agents. This fungus has the ability to control many plant pathogens (Waghunde et al., 2016). Mutations were used to improve the efficiency of Trichoderma fungus as a biocontrol agent against pathogenic fungi. Afterwards, derived mutants were compared with wild isolates in respect to antagonistic properties; and finally some isolates have been introduced with higher antagonistic effect than the wild isolate. Genes play a major role in the biocontrol cycle by controlling certain signals and leading to the secretion of certain enzymes or proteins that enable the pathogens to degrade and hence are known as biocontrol genes. Increased gene expression helps in enhanced biocontrol activity which helps to promote plant growth and prevents the plant from attacking pathogen. For commercial applications, therefore, the biocontrol genes can be cloned and generated in large amounts (Massart and Jijakli, 2007). Several approaches including chemical mutation, UV irradiation and genetic engineering to obtain enhanced cellulase producing strains have been given a high priority in the last decade (Kotchoni and Shonukan, 2002). Industrial application success for improved strains depends on their genetics and physiological characterization with a system that allows quick diagnosis. These diagnostic procedures for mutation are based upon a number of techniques in which resistant type of mutants can also be used for this purpose. Random mutagenesis methods are simple and easy to operate, though they have limitations, such as lack of stability. Mutagenesis is one such approach that induces diversification of the genetic structure of targeted organisms. There are several reports indicating the overproduction of cellulase by UV mutant strains of A. niger (Kang et al., 2004 and Nicolás-Santiago et al., 2006). These studies revealed that the UV exposure time and distance of the microorganism to the UV source are the main parameters which influence the yield of cellulase production. The aim of this study was to induce mutants using chemical mutagens and UV and evaluation of their antagonistic potential against the soil-borne fungal pathogens. Resolve the genetic variability of mutant derivatives with their wild strain using ISSR markers and isolation of the cbh1 gene, encoding for the exo-cellobiohydrolas from Trichoderma and their mutants and confirmed by PCR using specific primers.
Reference
Anees M, Tronsmo A,
Edel-Hermann V, Hjeljord L, Heraud C, Steinberg C. 2010. Characterization
of field isolates of Trichoderma antagonistic against Rhizoctonia
solani. Fungal Biology 114, 691-701.
Bastakoti S, Belbase S, Manandhar S, Arjyal C. 2017. Trichoderma species
as biocontrol agent against soil borne fungal pathogens. Nepal Journal of
Biotechnology 5, 39-45.
Bornet B, Goraguer F,
Joly G, Branchard M. 2002. Genetic diversity in European and Argentinian
cultivated potatoes (Solanum tuberosum subsp. tuberosum) detected by
inter-simple sequence repeats (ISSRs). Genome 45, 481-484.
Bose JL. 2014.
Chemical and UV mutagenesis. In: Bose J., editor. Vol. 1373. Humana Press; New
York, NY. The Genetic Manipulation of Staphylococci. Methods in Molecular
Biology.
Bryan G, Daniels T,
Osbourn A. 1995. Comparison of fungi within the Gaeumannomyces and Phialophora
complex by analysis of ribosomal DNA sequence. Applied and Environmental
Microbiology 61, 681-689.
Castrillo M, Bich G,
Amerio N, Rodríguez M, Zapata D, Villalba L. 2021. Assessment of cellulase
complex secretory capacity of Trichoderma strains and morphological
and molecular identification of the isolate with the highest enzymatic
secretion capacity. Journal Microbiology, Biotechnology and Food Sciences 10, 1-13.
Chand P, Aruna A,
Maqsood A, Rao L. 2005. Novel mutation method for increased cellulase
production. Journal Applied Microbiology 98, 318-323.
Daguerre Y, Siegel K,
Edel-Hermann V, Steinberg C. 2014. Fungal proteins and genes associated
with biocontrol mechanisms of soilborne pathogens: A review. Fungal
Biology Reviews 28, 97-125.
Florencio C, Couri S,
Sanchez F. 2012. Correlation between agar plate screening and solid-state
fermentation for the prediction of cellulase production by Trichoderma strains.
Enzyme Research 1-8.
Gams W, Bissett
J. 2002. Morphology and identification of Trichoderma. In: Kubicek,
C. P. and Harman, G. E. (Eds.). Trichoderma and Gliocladium:
Basic biology, taxonomy and genetics. Taylor & Francis Ltd 3-31.
Hermosa M, Keck E,
Chamorro I, Rubio B, Sanz L, Vizcaíno J, Grondona I, Minte E. 2004.
Genetic diversity shown in Trichoderma biocontrol isolates. Mycology
Research 108, 897-906.
Hibbett D. 1992.
Ribosomal RNA and fungal systematics. Transactions of the British Mycological
Society 33, 533-556.
Ikehata H, Ono T. 2011.
The mechanisms of UV mutagenesis. Journal of Radiation
Research 52, 115-125.
Kallinen K. 2016.
Behavioral inhibition and activation personality traits moderate safety culture
and risk behavior pp. 1-4. (Proc. Eur. Conf. Cogn. Ergon. ECCE 16).
Kang W, Park S, Lee S,
Hong I, Kim W. 2004. Production of cellulases and hemi cellulases by Aspergillus
niger KK2 from lignocellulosic biomass. Bioresour Technol 91, 153-156.
Kasana C, Salawan R,
Dhar H, Dutt S, Gulati A. 2008. A rapid and easy method for the detection
of microbial celluloses on agar plates using gram’s iodine. Current
Microbiology 57, 503-507.
Kotchoni S, Shonukan
O. 2002. Regulatory mutations affecting the synthesis of cellulose
in B. pumilus. World Journal of Microbiology and Biotechnology 18, 487-491.
Li H, Yang J, Roy B, Park
Y, Jiang J, Wang D, Miao G. 2010. Enhanced cellulase production of
the Trichoderma viride mutated by microwave and ultraviolet.
Microbiological Research 165, 190-198.
Massart S, Jijakli
M. 2007. Use of molecular techniques to elucidate the mechanisms of action
of biocontrol agents: A review. Journal Microbiological Methods 69, 229-241.
Nicolas-Santiago D,
Regalado-González C, García-Almendárez B, Fernández J, Téllez-Jurado A,
Huerta-Ochoa S. 2006. Physiological, morphological, and mannanase
production studies on Aspergillus niger uam-gs1 mutants. Electronic
Journal of Biotechnology 9, 50-60.
Nodvig S, Nielsen B,
Kogle E, Mortensen H. 2015. A CRISPR-cas9 system for genetic
engineering of filamentous fungi. PLoS One 10, e0133085.
Papavizas C, Lumsden
D. 1980. Biological control of soil borne fungal propagules. Annual
Review of Phytopathology 18, 389 – 413.
Rahman M, Rahman M,
Azad A, Alam M. 2011. Inhibitory effect of different plant extracts
and antifungal metabolites of Trichoderma strains on the conidial
germination and germ tube growth of Colletotrichum capsici causing
chili anthracnose. International Journal of Agronomy and Agricultural
Research 1, 20-28.
Samuels J, Dodd L, Gams
W, Castlebury A, Petrini O. 2002. Trichoderma species associated
with the green mold epidemic of commercially grown Agaricus bisporus.
Mycologia 94, 146-170.
Shawky T, Hickisch
B. 1984. Celluloytic activity of Trichoderma sp. Strain G, grown
on various cellulose substrates. Zbl. Mikrobiol 139, 91-96.
Snedecor W, Cochran
G. 1989. Statistical methods, 8th edn. Iowa State University Press,
Ames
Tamura K, Peterson D,
Peterson N, Stecher G, Nei M, Kumar S. 2011. Molecular evolutionary
genetics analysis using maximum likelihood, evolutionary distance, and maximum
parsimony methods. Molecular Biology and Evolution 28, 2731-2739.
Tulipa D, Roy A. 2022.
Antagonistic Property of the Stable Trichoderma Mutants against Fusarium
oxysporum f.sp. Lentis and Rhizoctonia solani. Environment and
Ecology 40, 1476-1480.
Waghunde R, Shelake M,
Sabalpara N. 2016. Trichoderma: A significant fungus for agriculture
and environment. African Journal of Agricultural Research 11, 1952-1965.
Walnuj A, Jhon P. 2013.
Role of mutation in Trichoderma spp. Rashtriya Krishi 8, 68
Wang L, Yen H, Shih L,
Chang C, Chang T, Wu C, Chai D. 2003. Production of xylanases from rice
bran by Streptomyces actuosus A-151. Enzyme and Microbial
Technology 33, 917- 925.
White J, Bruns T, Lee
S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal
RNA genes for phylogenetics. PCR Protocols. A Guide to Methods and
Applications 18, 315-322.
Yasui A, Kanno S, Takao
M. 2003. DNA damage and repair. Nihon Ronen Igakkai zasshi Japanese
Journal of Geriatrics 58, 577-580.
Zhang J, Zhong Y, Zhao
X, Wang T. 2010. Development of the cellulolytic fungus Trichoderma
reesei strain with enhanced β-glucosidase and filter paper activity using
strong artifical cellobiohydrolase 1 promoter. Bioresource
Technology 101, 9815-9818.
Source : Induction and characterization of a cellulase overproducing mutant strains of Trichodermaharzianum










%20in%20full.JPG)


0 comments:
Post a Comment