Mwatabu M. Edward, Were J. Omondi , Chiveu J. Chemulanga and Ochieng E. Ouma from the different institute of the Kenya, wrote a research article about Fungal Isolates: Inhibitory Indices Against Mycotoxins, about, In-vitro Inhibitory Indices of Selected Fungal Isolates against Mycotoxin Fungi. This research paper published by the International Journal of Mycrobiology and Mycology | IJMM.an open access scholarly research journal on Mycrobiology under the affiliation of the International Network For Natural Sciences | INNSpub. an open access multidisciplinary research journal publisher.
Abstract
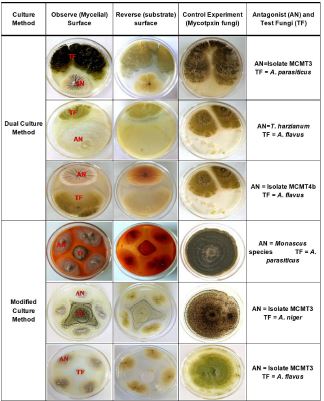
Limited fungal-based biocontrol products are available for use against mycotoxins in food and feed industry in Kenya. In filling this gap, in-vitro inhibitory assessment of six mycotoxin and nine non-mycotoxin species isolated from Western Kenya were placed on growth media using dual and modified plating techniques to determine the percentage inhibitions, capacity to form inhibition zones and degree of general antagonism on growth of mycotoxin fungi. The cultures were incubated at 25-27oC under 12-hour dark and 12-hour light conditions aseptically. Observations were made 10 days after incubation. Fungal isolates tested for their antagonistic effect on mycotoxin fungi were MCMT4b, MCMT3, MCHB2, T. harzianum, Monascus species, Biatrospora species, P. endophytica, C. olivaceum, and Epichloe species. Mycotoxin fungi tested were A. flavus, A. parasiticus, A. nomius, P. corrylophillum, P. auratiogriseum and A. niger. More than 80% growth inhibitory indices against mycotoxin fungi were expressed by T. harzianum, MCMT3, MCMT4b and Monascus species. Also, MCMT3, MCMT4b and Monascus species formed the largest inhibition zones against mycotoxin fungi. Fungal isolates MCMT3, MCMT4b, Monascus species and T. harzianum have growth suppression effect against A. flavus, A. parasiticus, A. niger, P. corrylophillum, and P. auratiogriseum in-vitro. More elaborate identification of the unidentified fungi, genetic characterization and field efficacy assessments of these isolates is recommended.
Read more : Cacao-Infused Tinupig: Crafting & Packaging Innovation | InformativeBD
Introduction
Food and feed safety is a global challenge due to mycotoxin contamination in warm regions across the globe (Eshelli et al., 2018; Truong et al., 2022). It is a significant challenge to sustain quality food and feed production, especially in most areas of sub-Saharan Africa (Nleya et al., 2018). However, it is nearing a catastrophic level in Kenya, with the country now ranking high in terms of severity and frequency of mycotoxin poisoning, often with human fatality (Kimanya, 2015; Tan, 2020). Mycotoxins are of high importance because they contribute to grain nutritional and quality losses of up to I billion metric tons on world's agricultural produce yearly (Ayofemi Olalekan Adeyeye, 2020). For example, exposure of humans to aflatoxins at even at low levels can cause cancer and several other health complications, but death is often the result of high and acute level exposure (Awuchi et al., 2020; Muthomi, 2018). The mycotoxin problem cuts across the agricultural value chain, affecting farmers, traders, markets, and consumers (animals and humans) (Danso et al., 2018).
For sustainable management of these toxins around the world, very few approved biological control products are available to manage mycotoxins in grains at preharvest globally. For instance, in 2015, the first fungal biocontrol product, AflaSafe KE01TM, was developed for use in Kenya (Migwi et al., 2020). However, prior to efficacy testing of potential bio-control agents against mycotoxin-producing fungi, testing the target and non-target effect of fungal interactions between the toxin producers (toxigenic) and nonproducers (atoxigenic) is necessary (Degola et al., 2021; Mylroie et al., 2016).
For effective development of efficacious biocontrol agent, the abundance and distribution of fungi by their geographical location in three major crop-producing regions of Kenya were classified (Salano, 2015). However, since the aflatoxin problem is still persistent in Kenya, it is For effective development of efficacious biocontrol agent, the abundance and distribution of fungi by their geographical location in three major crop-producing regions of Kenya were classified (Salano, 2015). However, since the aflatoxin problem is still persistent in Kenya, it is essential to identify additional efficacious biocontrol agents (fungi) with broad spectrum activity against a wide range of mycotoxin fungi. Therefore, this study aimed at determining the in-vitro inhibitory capacities of selected fungal species against mycotoxin-producing fungal isolates obtained from Western Kenya.
Reference
Abdallah MF, Ameye M,
De Saeger S, Audenaert K, Haesaert G. 2018. Biological control of
mycotoxigenic fungi and their toxins: An update for the pre-harvest approach.
In Mycotoxins-impact and management strategies 1-31. https://doi.org/10.5772
/intechopen. 76342
Agboyibor C, Kong WB,
Chen D, Zhang AM, Niu SQ. 2018. Monascus pigments production, composition,
bioactivity and its application: A review. Biocatalysis and Agricultural
Biotechnology 16, 433-447. https://doi.org/
10.1016/ j.bcab.2018.09.012
Awuchi CG, Amagwula IO,
Priya P, Kumar R, Yezdani U, Khan MG. 2020. Aflatoxins in foods and feeds:
A review on health implications, detection, and control. Bull. Environ.
Pharmacol. Life Sci 9, 149-155. http://www.bepls.com
Ayofemi Olalekan
Adeyeye S. 2020. Aflatoxigenic fungi and mycotoxins in food: a
review. Critical reviews in food science and nutrition 60, 709-721. https://doi.org/10.1080
/10408398.2018.1548429
Bandyopadhyay R,
Ortega-Beltran A, Akande A, Mutegi C, Atehnkeng J, Kaptoge L. 2016.
Biological Control of Aflatoxins in Africa: Current Status and Potential
Challenges in the Face of Climate Change. World Mycotoxins Journal 9, 771-789. https://doi.org/10.3920/WMJ2016.2130
Bentil JA, Thygesen A,
Mensah M, Lange L, Meyer AS. 2018. Cellulase production by white-rot
basidiomycetous fungi: solid-state versus submerged cultivation. Applied
microbiology and biotechnology 102, 5827-5839. https://doi.org/ 10.1007
/s00253-018-9072-8
Danso JK, Osekre EA,
Opit GP, Manu N, Armstrong P, Arthur FH, McNeill SG. 2018. Post-harvest
insect infestation and mycotoxin levels in maize markets in the Middle Belt of
Ghana. Journal of Stored Products Research 77, 9-15. https://doi.org/10.1016/j.jspr.2018.02.004
Degola F, Spadola G,
Forgia M, Turina M, Dramis L, Chitarra W, Nerva L. 2021. Aspergillus goes
viral: ecological insights from the geographical distribution of the mycovirome
within an Aspergillus flavus population and its possible correlation with
aflatoxin biosynthesis. Journal of Fungi 7, 833.
Ekanayaka AH, Hyde KD,
Gentekaki E, McKenzie EHC, Zhao Q, Bulgakov TS. 2019. Preliminary
classification of Leotiomycetes. Mycosphere 10, 310–489. https://doi.org/ 10.5943
/mycosphere/10/1/7
Eshelli M, Qader MM,
Jambi EJ, Hursthouse AS, Rateb ME. 2018. Current status and future
opportunities of omics tools in mycotoxin research. Toxins 10, 433. https://doi.org /10.3390
/toxins10110433
Ghorbanpour M, Omidvari
M, Abbaszadeh-Dahaji P, Omidvar R, Kariman K. 2018. Mechanisms underlying
the protective effects of beneficial fungi against plant diseases. Biological
Control 117, 147-157. https://doi.org/10.1016/
j.biocontrol.2017.11.006
Horta MAC, Murad NF, de
Oliveira Santos E, dos Santos CA, Mendes JS, Brandão MM, de Souza AP. 2018.
Network of proteins, enzymes and genes linked to biomass degradation shared by
Trichoderma species. Scientific Reports 8, 1-11.https://doi.org/10.1038/s41598-018-19671-w
Kagot V, De Boevre M,
Landschoot S, Obiero G, Okoth S, De Saeger S. 2022. Comprehensive analysis
of multiple mycotoxins and Aspergillus flavus metabolites in maize from Kenyan
households. International Journal of Food Microbiology 363, 109502. https://doi.org/
10.1016/j.ijfoodmicro.2021.109502
Kim C, Jung H, Kim YO,
Shin CS. 2006. Antimicrobial activities of amino acid derivatives of
Monascus pigments. FEMS microbiology letters 264, 117-124. https://doi.org/10.1111/j.1574-6968.2006.00451.x
Kucuk C, Kyvanc
M. 2011. In-vitro Interactions and Fungal Populations Isolated from Maize
Rhizosphere. Journal of Biological Sciences 11, 492-495. https://www.jstage.jst.go.jp/article
/bio/25/2/25_113/_article/-char/ja/
Kumar R, Kundu A, Dutta
A, Saha S, Das A. 2020. Profiling of volatile secondary metabolites of Chaetomium
globosum for potential antifungal activity against soil borne
fungi. Journal of Pharmacognosy and Phytochemistry 9, 922-927.
Lass‐Flörl C, Perkhofer
S, Mayr A. 2010. In vitro susceptibility testing in fungi: a global
perspective on a variety of methods. Mycoses 53, 1-11. https://doi.org/10.1111/j.1439-0507.2009.
Liu L, Zhao J, Huang Y,
Xin Q, Wang Z. 2018. Diversifying of chemical structure of native Monascus
pigments. Frontiers in Microbiology 9, 3143.https://www.frontiersin.org/articles/10.3389
/fmicb.2018.03143/full
Maurya KM, Singh R,
Tomer A. 2014. In Vitro Evaluation of Antagonistic Activity of Pseudomonas
fluorescens Against Fungal Pathogen. Journal of Biopesticides 7, 43-46. http://www.jbiopest.com/users/LW8/efiles/vol_7_1_43-46.pdf
Maurya S. 2020.
Biological control a sustainable approach for plant diseases management: A
review. Journal of Pharmacognosy and Phytochemistry 9, 1514-1523.
https://www. phytojournal.com/archives/2020/vol9issue2/PartY/9-2-84-468.pdf
Migwi B, Mutegi C,
Mburu J, Wagacha J, Cotty P, Bandyopadhyay R, Manyong VM. 2020. Assessment
of willingness-to-pay for Aflasafe KE01, a native biological control product
for aflatoxin management in Kenya. Food Additives & Contaminants: Part
A 37, 1951-1962. https://doi.org/10.1080/19440049.2020.181757
Mungan MD, Alanjary M,
Blin K, Weber T, Medema MH, Ziemert N. 2020. ARTS 2.0: feature updates and
expansion of the Antibiotic Resistant Target Seeker for comparative genome
mining. Nucleic acids research 48, 546-552. https://doi.org/10.1093/nar/gkaa374
Muthomi J. 2018.
Aflatoxin Research in Kenya. University of Nairobi, Parklands, Nairobi. https://documents.pub/document/pdfaflatoxin-research-in-kenya-department-of-plant-james-w-muthomi-department.html?page=1
Mylroie JE, Ozkan S,
Shivaji R, Windham GL, Alpe MN, Williams WP. 2016. Identification and
quantification of a toxigenic and non-toxigenic aspergillus flavus strain in
contaminated maize using quantitative real-time pcr. Toxins 8, 15. https://doi.org/10.3390/toxins8010015
Nleya N, Adetunji MC,
Mwanza M. 2018. Current status of mycotoxin contamination of food
commodities in Zimbabwe. Toxins 10, 89. https://doi.org/10.3390/toxins10050089
Rahman MA, Begum MF,
Alam MF. 2009. Screening of Trichoderma Isolates as a Biological Control
Agent against Ceratocystis paradoxa Causing Pineapple Disease of Sugarcane.
Microbiology 37, 277-285. https://doi.org/
10.4489/myco. 2009.37.4.277
Salano NE. 2015. Characterization
of aflatoxins and toxigenic aspergillus in maize and soil from the eastern
region of Kenya. Doctoral Dissertation, Egerton University, Kenya. https://
www.researchsquare.com/article/rs-9585/ latest.
Schoch LC,
Lopez-Giralrdez F, Sung G, Townsend PJ. 2009. The Ascomycota Tree of Life:
A Phylum-wide Phylogeny Clarifies the Origin and Evolution of Fundamental
Reproductive and Ecological Traits. Systematic Biology 58, 224-239.
https://academic.oup.com /sysbio/article-abstract/58/2/224/1671572
Shenouda ML, Cox
RJ. 2021. Molecular methods unravel the biosynthetic potential of
Trichoderma species. RSC advances 11, 3622-3635. https://doi.org/10.1039/D0RA09627J
Tan K. 2020.
Aflatoxin and Its Toxic Tragedies in Kenya. Journal of Young
Investigators 38, 10-12. https://www.jyi.org/2020-august/2020/8/1/
aflatoxin-and-its-toxic-tragedies-in-kenya
Truong NN, Tesfamariam
K, Visintin L, Goessens T, De Saeger S, Lachat C, De Boevre M. 2022.
Associating multiple mycotoxin exposure and health outcomes: current
statistical approaches and challenges. World Mycotoxin Journal 1-8. https://doi.org/10.3920/ WMJ2022.
Van Der Werf W, Bianchi
F. 2022. Options for diversifying agricultural systems to reduce pesticide
use: Can we learn from nature? Outlook on Agriculture 51, 105-113. https://doi.org/
10.1177%2F00307270221077442
Wild C, Miller JD,
Groopman JD. 2016. Mycotoxin Control in Low-and Middle-income Countries.
World Health Organization, Geneva, Switzerland. https://pubmed.ncbi.nlm.nih.gov
Yadav AN, Kour D, Kaur
T, Devi R, Yadav N. 2020. Functional annotation of agriculturally
important fungi for crop protection: current research and future
challenges. Agriculturally Important Fungi for Sustainable Agriculture:
Functional Annotation for Crop Protection 2, 347-356. https://link.springer.com/chapter/10.1007
/978-3-030-48474-3_12
Source : In-vitro Inhibitory Indices of Selected Fungal Isolates against Mycotoxin Fungi










%20in%20full.JPG)


0 comments:
Post a Comment