Bonou Malomon Aimé , Hounsossou Cocou Hubert , Ayinon Epiphane Helou Kossi Armel, Dossou Julien, and Biaou Olivier from the different institute of the Benin, wrote a research article about Analyzing High-Grade Breast Cancer via Fractal Dimension, entitled,"High histological grade breast cancer morphological evaluation on mammogram using the box-counting fractal dimension"This research paper published by the International Journal of Biomolecules and Biomedicine | IJBB an open access scholarly research journal on Biomedicine, under the affiliation of the International Network For Natural Sciences | INNSpub, an open access multidisciplinary research journal publisher.
Abstract
To evaluate the
high-grade breast cancer morphological complexity on mammogram. We conducted a
retrospective study using an open source data got from figshare repository.
These anonymized data were collected and used for a study approved by the
institutional review board. Cranio-Caudal and Medio-lateral mammograms and
their tumor segmented images from 66 patients subdivided in two groups high
histological grade (n=23) low-grade (low and intermediate, n=41). From breast
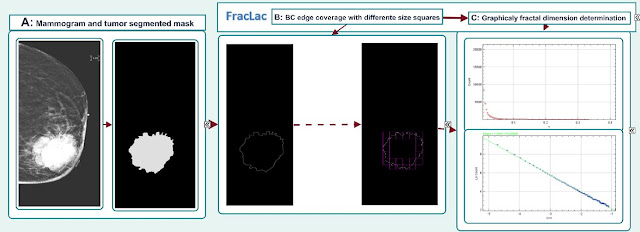
cancer image segmentation, we extracted fractal dimension using Fraclac,
plugin of ImageJ software based on box-counting method. For our
analysis we used comparatively the fractal dimension from cranio-caudal (CC)
and medio-lateral (MLO) images. We summarized the fractal dimension of our
cohort using boxplot and performed the Wilcoxon non-parametric statistic for
fractal dimension comparison of two groups (High-grade and low-grade). There
was not difference between CC (mean ± std= 1.1583±0.067) andmLO (mean ± std
=1.1551±0.055) breast cancer fractal dimension. For the high-grade
differentiation, CC andmLO images fractal dimension were contributed
respectively at a little difference but without statistically difference (P value=0.438
and 0.435). High-grade fractal dimensions mean were respectively 1.142±0.044
and 1.144±0.075 for CC andmLO images against 1.166±0.050 and 1.160±0.057 for
low-grade. It had been recorded a lower mean value of fractal dimension for
high-grade breast cancer without statistically significant. This finding shows
that the high-grade breast cancer tends to have a regular shape.
Read more : Saharan Flood Risk:Bechar Region Case Study | InformativeBD
Introduction
Breast cancer is the
most common cancer in women and a leading cause of cancer death worldwide (Bray
et al., 2018). Management of breast cancer relies on the availability of robust
clinical and pathological prognostic and predictive factors to guide patient
decision making and the selection of treatment. Histological grade is one of
important prognostic factor. It is based on the degree of differentiation of
the tumor tissue and based on the evaluation of three morphological features:
(a) degree of tubule or gland formation, (b) nuclear pleomorphism, and (c)
mitotic count. It is used to categorize breast cancer patients in three
clinical groups grade I (low), grade II (intermediate) and grade III (high)
(Elston and Ellis 1991). High-grade breast cancer is recognized as more
aggressive cancer type and is the worst survival prognostic and need a specific
treatment (WHO 2006; Rakha et al., 2008b, a).
To date, the
histological grading is one of popular method used to categorize breast cancer
patients in therapeutic groups (low and high risk). Whereas, this method has
been described as subjective method with sometimes inter-observer variability
(Gilchrist et al., 1985; Theissig et al., 1990).
In this context, some
authors attempted to describe the high-grade breast cancer aspect on medical
image in order to allow its a better identification for the clinician.
Regarding mammogram, Lamb et al. found that classical appearance of a low or
intermediate grade tumor is a speculated mass on mammography (Lamb et al., 2000).
SHIN et al. 2011 had also attempted to describe it morphological aspect on
mammogram because mammography is one of the primary breast imaging modalities
used in breast cancer diagnosis. They found that having Fairly slow developing
grade I tumors (low grade) and grade II tumors (intermediate grade) presents a
stroma reaction resulting in imaging by spicules while high grade with rapid
evolution, do not develop a stroma reaction and has a round shape (Shin et al.,
2011). The findings of both previous studies suggested that histological
high-grade breast cancer tends to have a particular margin.
Due to development the
Computer Aid Diagnosis (CAD) based on mammography several reliable quantitative
features had been used to describe breast cancer morphological characteristic.
In this context, shape factors such as compactness, fractional concavity,
spiculation index, and a Fourier-descriptor-based factor have been proposed for
breast lesion classification (Rangayyan et al. 1997, 2000). Latter fractal
dimension had been used in the same purpose and it allowed to get a result
better than with previous features for the breast cancer differentiation from
benign lesion (Rangayyan and Nguyen 2007). Fractal geometry is a powerful tool
for describing and modeling natural objects. Most of these applications employ
fractal dimension, a measure that captures the so-called complexity of the
object, a fundamental descriptor of analyzed objects represented in a digital
image. In this context, complexity expresses the level of detail detected at
different scales. This measure is immediately related to physical
characteristics, which are fundamental to the description and identification of
objects, even in our human vision system (texture analysis using fractal). In
last decade, following success of CAD, several studies used medical image
quantitative features in order to decrypt cancer biology (Sanduleanu et al., 2018).
Recently Fan et al. and Huang et al. extracted quantitative features from
medical image to find those which are relevant to breast cancer histological
grade (Huang et al., 2018; Fan et al., 2019). In these previous studies,
fractal dimension was not used, while it showed a better potential for the
differentiation of the breast tumors in according to their margin
characteristic. Based on hypothesis that the high-grade breast cancer presents
a particular margin, we used in this study, the fractal dimension to evaluate
its morphological complexity on mammogram and find the importance of this
quantitative feature in its differentiation from other grades (low and
intermediate).
Reference
Abràmoff DMD, Magalhães
Dr PJ, Ram Dr SJ. 2004. Image Processing with ImageJ 7.
Bray F, Ferlay J,
Soerjomataram I, Siegel RL, Torre LA, Jemal A. 2018. Global cancer
statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA: A Cancer Journal for Clinicians 68, 394-424.
Elston CW, Ellis IO. 1991.
Pathological prognostic factors in breast cancer. I. The value of histological
grade in breast cancer: experience from a large study with long-term follow-up.
Histopathology 19, 403-410.
Fan M, Liu Z, Xie S, Xu
M, Wang S, Gao X, Li L. 2019. Integration of dynamic contrast-enhanced
magnetic resonance imaging and T2-weighted imaging radiomic features by a
canonical correlation analysis-based feature fusion method to predict
histological grade in ductal breast carcinoma. Physics in Medicine & Biology 64, 215001.
Gilchrist KW, Kalish L,
Gould VE, Hirschl S, Imbriglia JE, Levy WM, Patchefsky AS, Penner DW, Pickren
J, Roth JA, Schinella RA, Schwartz IS, Wheeler JE. 1985. Interobserver
reproducibility of histopathological features in stage II breast cancer. Breast Cancer
Research and Treatment 5, 3-10.
Huang S, Franc BL,
Harnish RJ, Liu G, Mitra D, Copeland TP, Arasu VA, Kornak J, Jones EF, Behr SC,
Hylton NM, Price ER, Esserman L, Seo Y. 2018. Exploration of PET and MRI
radiomic features for decoding breast cancer phenotypes and prognosis. NPJ
Breast Cancer 4.
Lamb PM, Perry NM,
Vinnicombe SJ, Wells CA. 2000. Correlation Between Ultrasound
Characteristics, Mammographic Findings and Histological Grade in Patients with
Invasive Ductal Carcinoma of the Breast. Clinical Radiology 55, 40-44.
Rakha EA, El-Sayed ME,
Lee AHS, Elston CW, Grainge MJ, Hodi Z, Blamey RW, Ellis IO. 2008a.
Prognostic significance of Nottingham histologic grade in invasive breast
carcinoma. Journal of Clinical Oncology: Official Journal of the American
Society of Clinical Oncology 26, 3153-3158.
Rakha EA, El-Sayed ME,
Powe DG, Green AR, Habashy H, Grainge MJ, Robertson JFR, Blamey R, Gee J,
Nicholson RI, Lee AHS, Ellis IO. 2008b. Invasive lobular carcinoma of the
breast: response to hormonal therapy and outcomes. European Journal of Cancer
(Oxford, England: 1990) 44. 73-83.
Rangayyan RM,
El-Faramawy NM, Desautels JE, Alim OA. 1997. Measures of acutance and
shape for classification of breast tumors. IEEE transactions on medical
imaging 16, 799-810.
Rangayyan RM, Mudigonda
NR, Desautels JEL. 2000. Boundary modelling and shape analysis methods for
classification of mammographic masses. Medical and Biological Engineering and
Computing 38, 487-496.
Rangayyan RM, Nguyen
TM. 2007. Fractal Analysis of Contours of Breast Masses in Mammograms.
Journal of Digital Imaging 20, 223-237.
Sanduleanu S, Woodruff
HC, Jong EEC, de, Timmeren JE, van, Jochems A, Dubois L, Lambin P. 2018.
Tracking tumor biology with radiomics: A systematic review utilizing a
radiomics quality score. Radiotherapy and Oncology 127, 349-360.
Schneider CA, Rasband
WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis.
Nature Methods 9, 671-675.
Shin HJ, Kim HH, Huh
MO, Kim MJ, Yi A, Kim H, Son BH, Ahn SH. 2011. Correlation between
mammographic and sonographic findings and prognostic factors in patients with
node-negative invasive breast cancer. The British Journal of Radiology 84, 19-30.
Stavros AT. 2004. Breast
Ultrasound. Lippincott Williams & Wilkins.
Stavros AT, Thickman D,
Rapp CL, Dennis MA, Parker SH, Sisney GA. 1995. Solid breast
nodules: use of sonography to distinguish between benign and malignant lesions.
Radiology 196, 123-134.
Tamez-Peña J-G,
Rodriguez-Rojas J-A, Gomez-Rueda H, Celaya-Padilla J-M, Rivera-Prieto R-A,
Palacios-Corona R, Garza-Montemayor M, Cardona-Huerta S, Treviño V. 2018.
Radiogenomics analysis identifies correlations of digital mammography with
clinical molecular signatures in breast cancer. PLOS ONE 13, e0193871.
Theissig F, Kunze KD,
Haroske G, Meyer W. 1990. Histological Grading of Breast Cancer:
Interobserver, Reproducibility and Prognostic Significance. Pathology –
Research and Practice 186, 732-736.
Trevino V. 2018.
Breast Cancer Images & Segmentation – Correlation of Gene Expression
Subtypes and Image Features.
WHO. 2006.
Guidelines for management of breast cancer. World Health Organization, Regional
Office for the Eastern Mediterranean, Cairo.










%20in%20full.JPG)


0 comments:
Post a Comment