Krishnamurthy Yogesh, and Mididoddi Venkateshwarlu from the different institute of the india wrote a research article about, Assessing Carbosulfan's Neuro-genotoxic Impact on Carp Fish, entitled, Neuro-genotoxicity assessment of sublethal exposure of carbosulfan to freshwater Fish, Cyprinus carpio (L.). This research paper published by the Journal of Biodiversity and Environmental Sciences|JBES an open access scholarly research journal on Biodiversity, under the affiliation of the International Network For Natural science | INNSpub, an open access multidisciplinary research journal publisher.
Abstract
Carbosulfan, a
carbamate pesticide extensively employed in rural communities, enters the
aquatic environment by the proximity of agricultural lands to water bodies or
through direct application in such environments. The study’s goal was to
investigate the neurotoxic effects of carbosulfan using ACh and AChE levels in
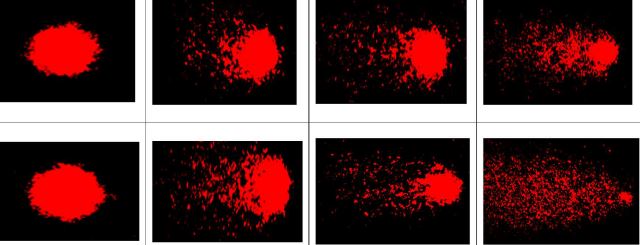
brain tissue, and genotoxic effects using Micronucleus (MN) assay in bloodcells and Comet assay in gill cells of Cyprinus carpio. The fish was
exposed to 1/5th & 1/10th sublethal concentrations of 96 h LC50 for
7, 14, and 21 days. There were significant (P<0.05) alterations in ACh and
AChE content and carbosulfan was induced to show MN formation and DNA damage in
concentration and time-dependent manner. The reduced ionic composition in C.carpio brain
tissue may explain the inhibition of AChE and the rise of ACh concentration.
The significant increase in MN and DNA damage observed in carbosulfan-exposed
fishes in the current study indicates the mutagenic/genotoxic potential of
carbosulfan in the freshwater fish C.carpio, as well as the potential
value of the Common carp for assessing pesticide pollution of freshwater
bodies. Changes in these characteristics may provide an early warning signal
for determining pesticide toxicity and its impact on aquatic species. As a
result, it is necessary to monitor the aquatic system and forecast the
hazardous effect of carbosulfan on fish; precautions should be taken while
using even low concentrations of carbosulfan, and prohibiting or restricting
carbosulfan usage is preferable.
Read more: Analyzing High-GradeBreast Cancer via Fractal Dimension | InformativeBD
Introduction
Carbosulfan is an insecticide used for the control ofinsects, mites, and nematodes on potatoes, sugarbeets, rice, maize, and citrus. It is exceedingly toxic tofish, and its toxicity is mediated in the nervous systemthrough acetylcholinesterase inhibition (Yi et al.,2006). Carbosulfan, which is commonly used in ruralcommunities, penetrates the aquatic environment bythe proximity of agricultural lands to water bodies ordirectly through reckless application in suchenvironments and affects aquatic species, and itsconcentrations in surface and groundwater arepredicted to be between 29μg/L and 0.64μg/L(Leppert et al., 1983; Sao et al., 2008). It is convertedto carbofuran in animals by hydroxylation oroxidation processes in water (Giri et al., 2003) andhas been restricted or banned in some countries,primarily due to the formation of highly toxicmetabolites. Pollution from pesticides in water killsfish and other aquatic creatures (Svensson et al.,1994). Fish are particularly sensitive toenvironmental changes. Thus, fish health may reflectand be an excellent predictor of the overall health ofan aquatic environment (Burkepile et al., 2000).Even though carbosulfan is not stable in water anddoes not remain in the environment, fishbioaccumulate to some amount due to their slowermetabolism. Its high water solubility, widespread usein the environment, and exposure to non-targetcreatures may all offer long-term risks to aquaticorganisms (IPCS, 1986).
The present study investigates the neurotoxic effectsof carbosulfan using ACh and AChE levels in braintissue, and genotoxic effects using Micronucleus(MN) assay in blood cells and Comet assay in gill cellsof Cyprinus carpio exposed in vivo. Because of thereduction of its activity, acetylcholinesterase (AChE;enzyme classification 3.1.1.7) is widely recognized as aparticular biomarker of carbamate pesticides(Fairbrother and Bennett, 1988). By hydrolyzing theubiquitous neurotransmitter acetylcholine, thisenzyme modulates neuronal transmission in thesynaptic cleft. AChE deficiency causes central andperipheral nervous system problems as well asmortality (Quinn, 1987).
Many studies have shown that the micronucleus(MN) test and the comet assay (CA) are the two most sensitive, rapid, and widely used methods for detecting the genotoxicity of chemicals and xenobiotics in the field and laboratory (Ateeq et al.,2002; Pandey et al., 2006). Despite the fact that carbosulfan has been shown to induce micronuclei, sister chromatid exchange, and chromosomal aberrations in human peripheral blood lymphocyte sand rat bone marrow cells (Sterhrer-Schmid and Wolf, 1995; Topaktas et al., 1996; Rencüzogullari and Topaktas, 2000; Giri et al., 2003), research on the genotoxic properties of carbosulfan in aquatic organisms is few, particularly data on its effects onfish. The experimental fish, Cyprinus carpio (L.) is acool to temperate water fish species that, due to its economic importance and status as a major element of many food chains around the world, is an ideal model indicator for toxicological investigations.
Reference
Adedeji OB, Adedeji AO,
Adeyemo OK, Agbede SA. 2020. Acute toxicity of diazinon to the African
catfish (Clarias gariepinus). Afr. J. Biotechnol 7(5), 651-54.
Adinarayan D, Kishore
S. 2018. Effect of deltamethrin in the Indian major carp Ciprinus
carpio with special reference to cholinergic activities. Int. J. Recent
Sci. Res 9(2), 24091-96. http://dx.doi.org/10.24327/ijrsr.2018.0902.1595
Al-Sabti K, Metcalfe
CD. 1995. Fish micronuclei for assessing genotoxicity in water. Mutat.
Res 343(2-3), 121-35. https://doi.org /10.1016 /0165-1218(95)90078-0
Altinok I, Capkin E,
Boran H. 2012. Mutagenic, genotoxic and enzyme inhibitory effects of
carbosulfan in rainbow trout Oncorhynchus mykiss, Pestic. Biochem.
Phys 102(1), 61-7 https://doi.org /10.1016 /j.pestbp.2011.10.011
Anderson D, Yu TW,
Philips BJ, Schmerzer P. 1994. The effects of various antioxidants and
other modifying agents on oxygen radical-generated DNA damage in human
lymphocytes in COMET assay. Mutat. Res. 307(1), 261-71.
APHA, AWWA, WPCP. 2005.
Standard methods for the examination of water and wastewater. 21 ed. American
Public Health Association, Washington, DC.
Ateeq B, Abul-Farah M,
Ali MN, Ahmad W. 2002. Induction of micronuclei and erythrocyte
alterations in the catfish Clarias batrachus by 2,4-
dichlorophenoxyacetic acid and butachlor. Mutat. Res. 518(2), 135-44. https://doi.org/10.1016/s1383-
Augustinson KB, In:
Glick D. (Ed.). 1957. Methods in Biochemical Analysis vol. 5. Interscience
Publishers, New York.
Ayllon F,
Garcia-Vasquez E. 2000. Induction of micronuclei and other nuclear
abnormalities in European minnow Phoxinus phoxinus and mollie Poecilia
latipinna: an assessment of the fish micronucleus test. Mutat. Res 467(2), 177-86. https://doi.org/10.1016/s1383-5718(00)00033-4
Belpaeme K, Delbeke K,
Zhu L, Kirsch-Volders M. 1996. Cytogenetic studies of PCB77 on brown trout
(Salmo trutta fario) using the micronucleus test and the alkaline comet assay,
Mutagenesis 11(5), 485-92. https://doi.org/10.1093
/mutage/11.5.485
Burkepile DE, Moore MT,
Hollandmm. 2000. Susceptibility of five nontarget organisms to aqueous
diazinon exposure. Bull. Environ. Contam. Toxicol. 64(1), 114-21. https://doi.org/10.1007/ s0012899
Cadet J, Douki T,
Gasparutto D, Ravanat JL. 2003, Oxidative damage to DNA: formation,
measurement and biochemical features. Mutat. Res. 531(1-2), 5-23. https://doi.org/10.1016/j.mrfmmm.
Capkin E, Boran H,
Altinok I. 2014.Response of Acetylcholinesterase (AChE) in the Erythrocyte
and Liver of Rainbow Trout Exposed to Carbosulfan. Turkish J. Fish Aquat.
Sci. 14, 643-50
Corbett JR. 1974.
The Biochemical Mode of Action of Pesticides. Academic Press. 330p.
Fairbrother A, Bennett
JK. 1988. The usefulness of cholin-esterase measurements. J. Wild
Dis. 4, 587-90.
Finney DJ. 1971.
Probit Analysis. Cambridge University Press, Cambridge 333p.
Giri S, Giri A, Sharma
GD, Prasad SB. 2003. Induction of sister chromatid exchanges by
cypermethrin and carbosulfan in bone marrow cells of mice in vivo.
Mutagenesis 18(1), 53-8
Grisolia CK, Cordeirocm
T. 2000. Variability in micronucleus induction with different mutagens
applied to several species of fish. Genet. Mol. Biol. 23(1), 235-39. https://doi.org/10.1590/S1415-47572
Hulbert AJ, Pamplona R,
Buffenstein R, Buttemer WA. 2007. Life and death: metabolic rate, membrane
com-position, and life span of animals. Physio. Rev 87(4), 1175-213.
https:// doi.org/ 10.1152 /physrev.00047.2006
International programme
on chemical safety (IPCS). 1986. Carbamate Pesticide: A General
Introduction. Environmental Health Criteria 64. Office of Publications, World
Health Organization, Geneva, Switzerland.
Jayashree IV,
Vijayalakshmi KK, Rahiman MA. 1994. The genotoxicity of Hinosan, an
organophosphorous pesticide in the in vivo mouse. Mutat. Res 322(2), 77-85. https://doi.org/10.1016
/0165-1218(94)00011-5
Kaushal BT, Misha A. 2013.
Investigation of acute toxicity of cadmium on snakehead fish Channa
punctatus – a comparative toxicity analysis on median lethal
concentration. Int. J. Adv. Biol. Res 3(2), 289-94.
Klaude M, Eriksson S,
Nygren J, Ahnstrom G. 1996. The comet assay: mechanisms and technical
considerations. Mutat. Res 363(2), 89-96. https:// doi.org
/10.1016/0921-8777(95)00063-1
Leppert BC, Markle JC,
Helt RC, Fujie GH. 1983. Determination of carbosulfan and carbofuran
residues in plants, soil, and water by gas chromatography. J. Agric. Food
Chem 31(2), 220-3. https://doi.org/10.1021/jf00116a009
Lowry OH, Rosebrough,
NJ, Farr AL, Randall RJ. 1951. Protein Measurement with the Folin Phenol
Reagent. J. Biol. Chem. 193(1), 265-75.
Matter BE, Grauwiler J. 1974.
The micronucleus test as a simple in vivo model, for the evaluation
of drug induced chromosomal aberrations, Comparative studies with 13 compounds.
Mutat. Res 29, 198-99.
Metcalf RL. 1951.
In: Glick, D. (Ed.), Methods in Biochemical Analysis vol. 5. Interscience
Publishers, New York.
Mitchelmore CL, Chipman
JK. 1998. DNA strand breakage in aquatic organisms and the potential value
of the comet assay in environmental monitoring. Mutat. Res 399(2), 135-47. https://doi.org/10.1016
/s0027-5107(97)00252-2
Nwani CD, Lakra WS,
Nagpure NS, Kumar R, Kushwaha B, Srivastava SK. 2010. Mutagenic and
genotoxic effects of carbosulfan in freshwater fish Channa punctatus (Bloch)
using micronucleus assay and alkaline single-cell gel electrophoresis. Food
Chem. Toxicol 48(1), 202-208. https://doi.org /10.1016
/j.fct.2009.09.041
Nwani CD, Nagpure NS,
Kumar R, Kushwaha B, Kumar P, Lakra WS. 2011. Mutagenic and genotoxic
assessment of atra- zine-based herbicide to freshwater fish Channa
punctatus (Bloch) using micronucleus test and single cell gel
electrophoresis. Environ. Toxicol. Pharmacol. 31(2), 314-22. http://dx.doi.org/10.1016/j.etap.2010.12.001
O’Brien RD. 1967.
Insecticides. In: Action and Metabolism. Academic Press, New York.
Organization for
Economic Cooperation and Development (OECD). 1992. OECD Guidelines for the
Testing of Chemicals, Section 2, Test No. 203: Fish, Acute Toxicity Test.
https://doi.org /10.1787
Pandey S, Nagpure NS,
Kumar R, Sharma S, Srivastava SK, Verma MS. 2006. Genotoxicity evaluation
of acute doses of endosulfan to freshwater teleost Channa punctatus (Bloch)
by alkaline single-cell gel electrophoresis. Ecotoxicol. Environ. Saf 65(1), 56-61. https://doi.org/10.1016/
j.ecoenv.2005.
Quinn DM. 1987.
Acetylcholinesterase: Enzyme structure, reaction dynamics, and virtual
transition states. Chem. Rev 87, 955-79. https://doi.org /10.1021 /CR00081A005
Reddy PM, Philip GH,
Bashamohideen M. 1992. Regulation of AChE system of freshwater fish, Cyprinus
carpio under fenvalerate toxicity. Bull. Environ. Contam. Toxicol 48(1), 18-22.
https:// doi.org /10.1007 /bf00197478
Reinecke SA, Reinecke
AJ. 2004. The comet assay as biomarker of heavy metal genotoxicity in
earthworms. Arch. Environ. Contam. Toxicol. 46(2), 208-15. https://doi.org/10.1007/s00244-003-2253-0
Rencüzogullari E,
Topaktas M. 2000. Chromosomal aberrations in cultured human lymphocytes
treated with the mixtures of carbosulfan, ethyl carbamate and ethyl
methanosulfonate. Cytologia 65, 83-92.
Sao A, Pillai AK, Gupta
VK. 2008. Spectrophotometric determination of carbosulfan in environmental
samples. J. Sci. Ind. Res 67, 1088-91.
Singh NP, McCoy MT,
Tice RR, Schneider EL. 1988. simple technique for quantification of low
levels of DNA damage in individual cell. Exp Cell Res 175(1), 184-191. https://doi.org/10.1016/0014-4827(88)90265-0
Sterhrer-Schmid P, Wolf
HU. 1995. Genotoxic evaluation of three heterocyclic N-methylcarbamate
pesticides using the mouse bone marrow micronucleus assay and Saccharomyces
cerevisiae strain D7 and D61.M. Mutat. Res 345(3-4), 111-25. https://doi.org/10.1016/0165-1218(95)90047-0
Svensson BG, Hallberg
T, Nilsson A, Schutz A, Hagmar L. 1994. Parameters of immunological
competence subjects with high consumption of fish contaminated with persistent
organochlorine compounds. Int. Arch. Occup. Environ. Health 65(6), 351-8. https://doi.org/10.1007/bf00383243
Topaktas M,
Rencüzogullari E, Ila HB. 1996. In vivo chromosomal aberrations
in bone marrow cells of rats with marshal. Mutat. Res 371(3-4), 259-64.
Van der Kloot WG. 1956.
Cholinesterase and sodium transport by frog muscle. Nature 178, 366-67.
Wang Z, Zang C,
Rosenfeld JA, Schones DE, Barski A, Cuddapah S, Cui K, Roh TY, Peng W, Zhang
MQ, Zhao K. 2008. Combinatorial patterns of histone acetylations and
methylations in the human genome. Nat. Genet 40(7), 897-903. https://doi.org/10.1038/ng.154
Yi MQ, Liu HX, Shi XY,
Liang P, Gao XW. 2006. Inhibitory effects of four carbamate insecticides
on acetylcholinesterase of male and female Carassius auratus in
vitro. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 143(1), 1113-6.










%20in%20full.JPG)


0 comments:
Post a Comment